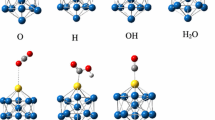
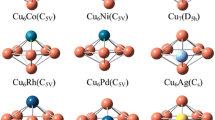
Abstract
The mechanism of water-gas shift reaction on the transition metal of Co, Ni, Cu (from the 3d row), Rh, Pd, Ag (from the 4d row), Ir, Pt, and Au (from the 5d row) @Cu12 bimetallic clusters have been studied using density functional theory (DFT) calculations. Three reaction mechanisms including redox, carboxyl, and formate mechanisms, which are equal to CO* + O* → CO2 (g), CO* + OH* → COOH* → CO2 (g) + H*, and CO* + H* + O* → CHO* + O* → HCOO** → CO2 (g) + H*, respectively, have been studied. The result revealed that the WGSR prefer to follow the carboxyl mechanism on the TM@Cu12 surfaces. The rate-controlling step of WGS reaction is H2O dissociation into OH and H or COOH decomposition into CO and OH. The transition metal additive in Cu cluster could enhance the activity of water dissociation, which is beneficial for WGS reaction. Especially, doping Ni has the largest promotion effect in reducing the active barrier, the reason is electronic effect. The calculation indicates that Ni@Cu12 is thus the promising candidates for improved WGSR catalysts. In addition, The TOF values are studied to estimate effectively activity of the TM@Cu12 cluster. To get insight into conclusion, reaction mechanism and structure of cluster was elucidated by the relative energy profiles and detailed electronic local density of states (LDOS).
Similar content being viewed by others
References
Song, W.Y. and Hensen, E.J.M., ACS Catal., 2014, vol. 4, p. 1885.
Azzam, K.G., Babich, I.V., Seshan, K., and Lefferts, L., Appl. Catal. B, 2008, vol. 80, p. 129.
Tao, F. and Ma, Z., Phys. Chem. Chem. Phys., 2013, vol. 15, p. 15260.
Yang, Z.X., Xie, L.G., Ma, D.W., and Wang, G.T., J. Phys. Chem. C, 2011, vol. 115, p. 6730.
Lin, C.H., Chen, C.L., and Wang, J.H., J. Phys. Chem. C, 2011, vol. 115, p. 18582.
Yang, L., Karim, A., and Muckerman, J.T., J. Phys Chem C, 2013, vol. 117, p. 3414.
Meunier, F.C., Reid, D., Goguest, A., Shekhtman, S., Hardacre, C., Burch, R., Deng, W., and Flytzani-Stephanopoulos, M., J. Catal., 2007, vol. 247, p. 277.
Gokhale, A.A., Dumesic, J.A., and Mavrikakis, M., J. Am. Chem. Soc., 2008, vol. 130, p. 1402.
Kalamaras, C.M., Panagiotopoulou, P., Kondarides, D.I., and Efstathiou, A.M., J. Catal., 2009, vol. 264, p. 117.
Kalamaras, C.M., Americanou, S., and Efstathiou, A.M., J. Catal., 2011, vol. 279, p. 287.
Aguila, G., Guerrero, S., and Araya, P., Catal. Commun., 2008, vol. 9, p. 2550.
Gokhale, A.A., Dumestic, J.A., and Mavrikakis, M., J. Am. Chem. Soc., 2008, vol. 130, p. 1402.
Tang, Q.L. and Liu, Z.P., J. Phys. Chem. C, 2010, vol. 114, p. 8423.
Tang, Q.L., Chen, Z.X., and He, X., Surf. Sci., 2009, vol. 603, p. 2138.
José, L.C., Fajín, M., Natália, D.S., Cordeiro, F., José, R.B., and Gomes, J., J. Catal., 2009, vol. 268, p. 131.
Wang, G., Jiang, L., Cai, Z., Pan, Y., Zhao, X., Huang, W., Xie, K., Li, Y., Sun, Y., and Zhong, B., J. Phys. Chem. B, 2003, vol. 107, p. 557.
Vidal, A.B. and Liu, P., Phys. Chem. Chem. Phys., 2012, vol. 14, p. 16626.
Fu, Q., Deng, W., Saltsburg, H., and Flytzani-Stephanopoulos, M., Appl. Catal. B, 2005, vol. 56, p. 57.
Lin, R.J., Chen, H.L., Ju, S.P., Li, F.Y., and Chen, H.T., J. Phys. Chem. C, 2012, vol. 116, p. 336.
Huang, S.C., Lin, C.H., and Wang, J.H., J. Phys. Chem. C, 2010, vol. 114, p. 9826.
Chen, Y.Y., Dong, M., Wang, J.G., and Jiao, H.J, J. Phys. Chem. C, 2012, vol. 116, p. 25368.
Wu, S.R., Lin, R.J., Jang, S.M., Chen, H.L., Wang, S.M., and Li, F.Y., J. Phys. Chem., C, 2014, vol. 118, p. 298.
Rodriguez, J.A., Catal. Today, 2011, vol. 160, p. 3.
Lin, J.H. and Guliants, V.V., Appl. Catal., A, 2012, vol. 445, p. 187.
Fu, Z.M., Wang, J.Q., Zhang, N., An, Y.P., and Yang, Z.X., Int. J. Hydrogen Energy., 2015, vol. 40, p. 2193.
Lin, C.H., Chen, C.L., and Wang, J.H., J. Phys. Chem. C, 2011, vol. 115, p. 18582.
Phatak, A.A., Delgass, W.N., Ribeiro, F.H., and Schneider, W.F., J. Phys. Chem. C, 2009, vol. 113, p. 7269.
Callaghan, C.A., Vilekar, S.A., Fishtik, I., and Datta, R., Appl. Catal. A, 2008, vol. 345, p. 213.
Liu, P., J. Chem. Phys., 2010, vol. 133, p. 204705.
Kim, H.Y., Lee, H.M., and Henkelman, G., J. Am. Chem. Soc., 2012, vol. 134, p. 1560.
Yang, X., Cheng, F., Tao, Z., and Chen, J., J. Power Sources, 2011, vol. 196, p. 2785.
Lin, J.H., Biswas, P., Guliants, V.V., and Misture, S., Appl. Catal., A, 2010, vol. 387, p. 87.
Allian, A.D., Takanabe, K., Fujdala, K.L., Hao, X., Truex, T.J., Cai, J., Buda, C., Neurock, M., and Iglesia, E., J. Am. Chem. Soc., 2011, vol. 133, p. 4498.
Gan, L.Y. and Zhao, Y.J., J. Phys. Chem. C, 2012, vol. 116, p. 16089.
Gan, L.Y., Zhang, Y.X., and Zhao, Y.J., J. Phys. Chem. C, 2010, vol. 114, p. 996.
Bian, J., Xiao, M., Wang, S.J., Lu, Y.X., and Meng, Y.Z., Catal. Commun., 2009, vol. 10, p. 1529.
Gan, L.Y., Tian, R.Y., Yang, X.B., Lu, H.D., and Zhao, Y.J., J. Phys. Chem. C, 2012, vol. 116, p. 745.
Kozuch, S. and Shaik, S., Acc. Chem. Res., 2010, vol. 44, p. 101.
Perdew, J.P., Burke, K., and Ernzerhof, M., J. Phys. Rev. Lett., 1996, vol. 77, p. 3865.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., et al., Gaussian 09, Revision A.1, Wallingford, CT: Gaussian, 2009.
Wadt, W.R. and Hay, P.J., J. Chem. Phys., 1985, vol. 82, p. 270
Hay, P.J. and Wadt, W.R., J. Chem. Phys., 1985, vol. 82, p. 299.
Wang, F., Zhang, D.J., Xu, X.H., and Ding, Y., J. Phys. Chem. C, 2009, vol. 113, p.18032.
Huber, K.P. and Herzberg, G., Constants of Diatomic Molecules, New York: van Nostrand Reinhold, 1979.
Amatore, C. and Jutand, A., J. Organomet. Chem., 1999, vol. 576, p. 254.
An, X., Guo, L., Li, A., Liu, N., and Cao, Z., Prot. Met. Phys. Chem. Surf., 2015, vol. 51, no. 5, p. 740.
Gunugunuri, K.R., Boolchand, P., and Panagiotis, G.S., J. Phys. Chem. C, 2012, vol. 116, p. 1109.
Guo, L. and Zhang, X., J. Phys. Chem. C, 2014, vol. 118, p. 533.
Zeinalipour-Yazdi, C.D. and Efstathiou, A.M., J. Phys. Chem. C, 2008, vol. 112, p. 19030.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Liu, N., Guo, L., Cao, Z. et al. Density functional theory study of water-gas shift reaction on TM@Cu12 core-shell nanoclusters. Prot Met Phys Chem Surf 52, 387–398 (2016). https://doi.org/10.1134/S2070205116030187
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205116030187