Abstract
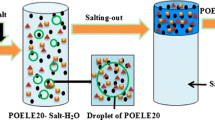
In conventional microextraction procedures, the disperser (organic solvent or ionic liquid) is left in the aqueous phase and discarded after finishing the microextraction process. Because the disperser is water-soluble, it results in low extraction recovery for polar compounds. In this investigation, an ionic-liquid-based microextraction (ILBME) was integrated with salting-out assisted liquid-liquid microextraction (SALLME) to build an ionic-liquid-based, salt-induced, dual microextraction (ILSDME) for isolation of five fluoroquinolone antibiotics (FQs) with high polarity (log P, −1.0 to 1.0). The proposed ILSDME method incorporates a dual microextraction by converting the disperser in the ILBME to the extractor in the SALLME. Optimization of key factors was conducted by integrating single-factor experiments and central composite design. The optimized experimental parameters were 80 μL [C8MIM][PF6] as extractor, 505 μL acetone as disperser, pH = 2.0, 4.1 min extraction time, and 4.2 g of Na2SO4. Under optimized conditions, high ERs (90.6–103.2 %) and low LODs (0.07–0.61 μg kg−1) were determined for five FQs in swine feed. Experimental precision based on RSDs was 1.4–5.2 % for intra-day and 2.4–6.9 % for inter-day analyses. The combination of ILBME with SALLME increased FQ recoveries by 15–20 % as compared with SALLME, demonstrating that the ILSDME method can enhance extraction efficiency for polar compounds compared to single-step microextraction. Therefore, the ILSDME method developed in this study has wide application for pretreatment of moderately to highly polar pollutants in complex matrices.

A dual microextraction was developed by integrating ionic-liquid-based microextraction with salting-out assisted liquid-liquid microextraction for isolation of five fluoroquinolone antibiotics (FQs) with high polarity (log P = −1.0 to 1.0). The principle of dual microextraction is based on converting the remaining disperser from the first microextraction into an extractor in the second microextraction. Single-factor experiment and central composite design were applied for optimizing operational parameters using 3D response surfaces and contour lines. Under optimized conditions, the method provided high extraction recoveries and low LODs for five FQs in swine feed. The prominent advantage of the dual microextraction is rapid and highly efficient extraction of moderately to highly polar fluoroquinolones from complex matrices





Similar content being viewed by others
References
Speltini A, Sturini M, Maraschi F, Profumo A, Albini A. Analytical methods for the determination of fluoroquinolones in solid environmental matrices. Trends Anal Chem. 2011;30:1337–50.
Gao SQ, Jin HY, Ding Y, Zhang N, Wang Y, Ren RB, et al. Ionic liquid-based homogeneous liquid-liquid microextraction for the determination of antibiotics in milk by high-performance liquid chromatography. J Chromatogr A. 2011;1218:7254–63.
Wang YS, Zhou LM, Zeng H. Relationships between mechanism of adverse reactions and structure for fluoroquinolones. J Adverse Drug Reac. 2005;6:405–7.
Wang L, Yang H, Zhang C, Mo Y, Lu X. Determination of oxytetracycline, tetracycline and chloramphenicol antibiotics in animal feeds using subcritical water extraction and high performance liquid chromatography. Anal Chim Acta. 2008;619:54–8.
Conti GO, Copat C, Wang Z, Agati P, Cristaldi A. Determination of illegal antimicrobials in aquaculture feed and fish: an ELISA study. Food Control. 2015;50:937–41.
Tossaint B, Chedin M, Vincent U, Bordin G, Rodriguez AR. Determination of fluoroquinolone antibiotic residues in pig kidney using liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2005;1088:40–8.
Huang XJ, Yuan DX, Lin QM. Preparation of cation-exchange stir bar sorptive extraction based on monolithic material and its application to the analysis of soluble cations in milk by ion chromatography. J Chromatogr A. 2011;1217:2667–73.
Hermo MP, Barron D, Barbosa J. Determination of residues of quinolones in pig muscle: comparative study of classical and microwave extraction techniques. Anal Chim Acta. 2005;539:77–82.
Sharifi V, Abbasi A, Nosrati A. Application of hollow fiber microextraction and dispersive liquid-liquid microextraction techniques in analytical toxicology. J Food Drug Anal. 2016;24:264–76.
Vazquez MM, Galera MM, Garcia MD. Determination of eight fluoroquinolones in groundwater samples with ultrasound-assisted ionic liquid dispersive liquid-liquid microextraction prior to high-performance liquid chromatography and fluorescence detection. Anal Chim Acta. 2012;748:20–7.
Vakh C, Pochivalov A, Andruch V, Moskvin L, Bulatov A. A fully automated effervescence-assisted switchable solvent-based liquid phase microextraction procedure: liquid chromatographic determination of ofloxacin in human urine samples. Anal Chim Acta. 2016;907:54–9.
Bruno AR, Bruno RBC, Nayara CPA, Anderson RMO, Juliana MOS, Maha AT, et al. A fast method for bisphenol A and six analogues (S, F, Z, P, AF, AP) determination in urine samples based on dispersive liquid-liquid microextraction and liquid chromatography-tandem mass spectrometry. Talanta. 2016;154:511–9.
Wu M, Wang LY, Zeng BZ, Zhao FQ. Ionic liquid polymer functionalized carbon nanotubes-doped poly(3,4-ethylenedioxythiophene) for highly-efficient solid-phase microextraction of carbamate pesticides. J Chromatogr A. 2016;1444:42–9.
Wang J, Huang SS, Wang P, Yang YL. Method development for the analysis of phthalate esters in tea beverages by ionic liquid hollow fibre liquid-phase microextraction and liquid chromatographic detection. Food Control. 2016;67:278–84.
Ebrahimpour B, Yamini Y, Moradi M. Application of ionic surfactant as a carrier and emulsifier agent for the microextraction of fluoroquinolones. J Pharm Biomed Anal. 2012;66:264–70.
Nerina BL, Paula B, Adrian C, Adrián GA, Néstor FC, Jorgelina CA. Ultrasound leaching–dispersive liquid–liquid microextraction based on solidification of floating organic droplet for determination of polybrominated diphenyl ethers in sediment samples by gas chromatography–tandem mass spectrometry. J Chromatogr A. 2013;1285:15–21.
Tsai WH, Chuang HY, Chen HH, Huang JJ, Chen HC, Cheng SH, et al. Application of dispersive liquid-liquid microextraction and dispersive micro-solid-phase extraction for the determination of quinolones in swine muscle by high-performance liquid chromatography with diode-array detection. Anal Chim Acta. 2009;656:56–62.
Myasein F, Kim E, Zhang J, Wu H, Tawakol A. Rapid, simultaneous determination of lopinavir and ritonavir in human plasma by stacking protein precipitations and salting-out assisted liquid/liquid extraction, and ultrafast LC-MS/MS. Anal Chim Acta. 2009;651:112–6.
Cai YQ, Cai YE, Shi YL, Liu JM, Mou SF, Lu YQ. A liquid-liquid extraction technique for phthalate esters with water-soluble organic solvents by adding inorganic salts. Microchim Acta. 2007;157:73–9.
Gao M, Wang HL, Ma MP, Zhang YN, Yin XH, Dahlgren RA, et al. Optimization of a phase separation based magnetic-stirring salt-induced liquid-liquid microextraction method for determination of fluoroquinolones in food. Food Chem. 2015;175:181–8.
Mohammadi A, Tavakoli R, Kamankesh M, Rashedi H, Attaran A, Delavar M. Enzyme-assisted extraction and ionic liquid-based dispersive liquid-liquid microextraction followed by high-performance liquid chromatography for determination of patulin in apple juice and method optimization using central composite design. Anal Chim Acta. 2013;804:104–10.
Ma WY, Lu YB, Hu RL, Chen JH, Zhang ZZ, Pan YJ. Application of ionic liquids based microwave-assisted extraction of three alkaloids N-nornuciferine, O-nornuciferine, and nuciferine from lotus leaf. Talanta. 2010;80:1292–7.
Magali K, Tiele MR, Renato Z. Optimization by central composite design of a modified quEChERS method for extraction of pesticide multiresidue in sweet pepper and analysis by ultra-high-performance liquid chromatography-tandem mass spectrometry. Food Anal Method. 2015;8:728–39.
Sereshti H, Izadmanesh Y, Samadi S. Optimized ultrasonic assisted extraction-dispersive liquid-liquid microextraction coupled with gas chromatography for determination of essential oil of Oliveria decumbens Vent. J Chromatogr A. 2011;1218:4593–8.
Diao XX, Ma ZY, Lei P, Zhong DF, Zhang YF, Chen XW. Enantioselective determination of 3-n-butylphthalide (NBP) in human plasma by liquid chromatography on a tecoplanin-based chiral column coupled with tandem mass spectrometry. J Chromatogr B. 2013;939:67–72.
Diao XX, Ma ZY, Wang HD, Zhong DF, Zhang YF, Jin J, et al. Simultaneous quantitation of 3-n-butylphthalide (NBP) and its four major metabolites in human plasma by LC-MS/MS using deuterated internal standards. J Pharm Biomed Anal. 2013;78-79:19–26.
Tufa RA, Pinacho DG, Pascual N, Granados M, Companyo R, Marco MP. Development and validation of an enzyme linked immunosorbent assay for fluoroquinolones in animal feeds. Food Control. 2015;57:195–201.
Janusch F, Scherz G, Mohring S, Hamscher G. Determination of fluoroquinolones in chicken feces—a new liquid-liquid extraction method combined with LC-MS/MS. Environ Toxicol Pharm. 2014;38:792–9.
Acknowledgments
This work was jointly supported by the China National Natural Science Foundation (21577107), the Zhejiang Provincial Public Benefit Project (2016C37019 and 2016C34011), the Zhejiang Provincial Natural Science Foundation (LY15B070009 and LY15B070010), and the Wenzhou International Cooperation Project (H20140004). The authors thank Professor Randy A. Dahlgren, employed in Davis, University of California, USA, for correcting this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Huili Wang and Ming Gao contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 450 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Gao, M., Gao, J. et al. Determination of fluoroquinolone antibiotics via ionic-liquid-based, salt-induced, dual microextraction in swine feed. Anal Bioanal Chem 408, 6105–6114 (2016). https://doi.org/10.1007/s00216-016-9719-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9719-1