Abstract
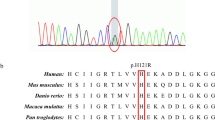
Mutations in the coiled-coil-helix-coiled-coil-helix domain-containing protein 10 gene (CHCHD10), involved in mitochondrial function, have recently been reported as a causative gene of amyotrophic lateral sclerosis (ALS). The aim of this study was to obtain the mutation prevalence of CHCHD10 and the phenotypes with mutations in Chinese ALS patients. A cohort of 499 ALS patients including 487 sporadic ALS (SALS) and 12 familial ALS (FALS), from the Department of Neurology, West China Hospital of Sichuan University, were screened for mutations of all exons of the CHCHD10 gene by Sanger sequencing. Novel candidate mutations or variants were confirmed by polymerase chain reaction-restriction fragment length polymorphism in 466 healthy individuals. All patients identified with mutations of CHCHD10 gene were screened for mutations of the common ALS causative genes including C9orf72, SOD1, TARDBP, FUS, PFN1, and SQSTM1. Three heterozygous variants, including two missense mutations (c.275A > G (p.Y92C) and c.306G > C (p.Q102H)) and a synonymous change c.306G > A (p.Q102Q), were found in exon 3 of CHCHD10 in three alive SALS individuals (with the longest disease duration of 8.6 years), all of which were not detected in healthy controls. No mutation in CHCHD10 was identified in FALS patients. No mutation was found in the aforementioned common ALS causative genes in the patients who carried CHCHD10 mutations. The mutation frequency of CHCHD10 (0.4 %, 2/487) in a Chinese SALS population suggests CHCHD10 gene mutation appears to be an uncommon cause of ALS in Chinese populations. CHCHD10 mutations are associated with a slow progression and long disease duration.

Similar content being viewed by others
References
Caballero-Hernandez D, Toscano MG, Cejudo-Guillen M, Garcia-Martin ML, Lopez S, Franco JM, Quintana FJ, Roodveldt C (2016) The ‘omics’ of amyotrophic lateral sclerosis. Trends Mol Med 22(1):53–67. doi:10.1016/j.molmed.2015.11.001
Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72(2):257–268. doi:10.1016/j.neuron.2011.09.010
Majounie E, Renton AE, Mok K, Dopper EGP, Waite A, Rollinson S, Chiò A, Restagno G et al (2012) Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol 11(4):323–330. doi:10.1016/s1474-4422(12)70043-1
Freischmidt A, Wieland T, Richter B, Ruf W, Schaeffer V, Muller K, Marroquin N, Nordin F et al (2015) Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat Neurosci 18(5):631–636. doi:10.1038/nn.4000
Renton AE, Chio A, Traynor BJ (2014) State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17(1):17–23. doi:10.1038/nn.3584
Andersen PM, Al-Chalabi A (2011) Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat Rev Neurol 7(11):603–615. doi:10.1038/nrneurol.2011.150
Blokhuis AM, Groen EJ, Koppers M, van den Berg LH, Pasterkamp RJ (2013) Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol 125(6):777–794. doi:10.1007/s00401-013-1125-6
Johann S, Heitzer M, Kanagaratnam M, Goswami A, Rizo T, Weis J, Troost D, Beyer C (2015) NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia 63(12):2260–2273. doi:10.1002/glia.22891
Cozzolino M, Ferri A, Valle C, Carri MT (2013) Mitochondria and ALS: implications from novel genes and pathways. Mol Cell Neurosci 55:44–49. doi:10.1016/j.mcn.2012.06.001
Cozzolino M, Rossi S, Mirra A, Carri MT (2015) Mitochondrial dynamism and the pathogenesis of amyotrophic lateral sclerosis. Front Cell Neurosci 9:31. doi:10.3389/fncel.2015.00031
Ajroud-Driss S, Fecto F, Ajroud K, Lalani I, Calvo SE, Mootha VK, Deng H-X, Siddique N et al (2014) Mutation in the novel nuclear-encoded mitochondrial protein CHCHD10 in a family with autosomal dominant mitochondrial myopathy. Neurogenetics 16(1):1–9. doi:10.1007/s10048-014-0421-1
Bannwarth S, Ait-El-Mkadem S, Chaussenot A, Genin EC, Lacas-Gervais S, Fragaki K, Berg-Alonso L, Kageyama Y et al (2014) A mitochondrial origin for frontotemporal dementia and amyotrophic lateral sclerosis through CHCHD10 involvement. Brain 137(Pt 8):2329–2345. doi:10.1093/brain/awu138
Dupuis L, Gonzalez de Aguilar JL, Oudart H, de Tapia M, Barbeito L, Loeffler JP (2004) Mitochondria in amyotrophic lateral sclerosis: a trigger and a target. Neurodegener Dis 1(6):245–254. doi:10.1159/000085063
Bannwarth S, Ait-El-Mkadem S, Chaussenot A, Genin EC, Lacas-Gervais S, Fragaki K, Berg-Alonso L, Kageyama Y et al (2015) Reply: CHCHD10 mutations in Italian patients with sporadic amyotrophic lateral sclerosis. Brain 138(8):e373–e373. doi:10.1093/brain/awu385
Muller K, Andersen PM, Hubers A, Marroquin N, Volk AE, Danzer KM, Meitinger T, Ludolph AC et al (2014) Two novel mutations in conserved codons indicate that CHCHD10 is a gene associated with motor neuron disease. Brain 137(Pt 12):e309. doi:10.1093/brain/awu227
Chaussenot A, Le Ber I, Ait-El-Mkadem S, Camuzat A, de Septenville A, Bannwarth S, Genin EC, Serre V et al (2014) Screening of CHCHD10 in a French cohort confirms the involvement of this gene in frontotemporal dementia with amyotrophic lateral sclerosis patients. Neurobiol Aging 35(12):2884e.2881–2884e.2884. doi:10.1016/j.neurobiolaging.2014.07.022
Johnson JO, Glynn SM, Gibbs JR, Nalls MA, Sabatelli M, Restagno G, Drory VE, Chio A et al (2014) Mutations in the CHCHD10 gene are a common cause of familial amyotrophic lateral sclerosis. Brain 137(Pt 12):e311. doi:10.1093/brain/awu265
Kurzwelly D, Krüger S, Biskup S, Heneka MT (2015) A distinct clinical phenotype in a German kindred with motor neuron disease carrying a CHCHD10 mutation: figure 1. Brain 138(9):e376–e376. doi:10.1093/brain/awv014
Zhang M, Xi Z, Zinman L, Bruni AC, Maletta RG, Curcio SAM, Rainero I, Rubino E et al (2015) Mutation analysis of CHCHD10 in different neurodegenerative diseases: figure 1. Brain 138(9):e380–e380. doi:10.1093/brain/awv082
Dols-Icardo O, Nebot I, Gorostidi A, Ortega-Cubero S, Hernandez I, Rojas-Garcia R, Garcia-Redondo A, Povedano M et al (2015) Analysis of the CHCHD10 gene in patients with frontotemporal dementia and amyotrophic lateral sclerosis from Spain. Brain 138:e400. doi:10.1093/brain/awv175
Jiao B, Xiao T, Hou L, Gu X, Zhou Y, Zhou L, Tang B, Xu J, Shen L (2015) High prevalence of CHCHD10 mutation in patients with frontotemporal dementia from China. Brain. awv367. doi:10.1093/brain/awv367
Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1(5):293–299
Kenna KP, McLaughlin RL, Byrne S, Elamin M, Heverin M, Kenny EM, Cormican P, Morris DW et al (2013) Delineating the genetic heterogeneity of ALS using targeted high-throughput sequencing. J Med Genet 50(11):776–783. doi:10.1136/jmedgenet-2013-101795
Wei Q, Chen X, Zheng Z, Huang R, Guo X, Cao B, Bak TH, Shang H (2015) Screening for cognitive impairment in a Chinese ALS population. Amyotroph Lateral Scler Frontotemporal Degener 16(1–2):40–45. doi:10.3109/21678421.2014.966311
Chen Y, Zheng ZZ, Huang R, Chen K, Song W, Zhao B, Chen X, Yang Y et al (2013) PFN1 mutations are rare in Han Chinese populations with amyotrophic lateral sclerosis. Neurobiol Aging 34(7):1922 e1921–1925. doi:10.1016/j.neurobiolaging.2013.01.013
Kumar P, Henikoff S, Ng PC (2009) Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4(8):1073–1081. doi:10.1038/nprot.2009.86
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR (2010) A method and server for predicting damaging missense mutations. Nat Methods 7(4):248–249. doi:10.1038/nmeth0410-248
Darshi M, Mendiola VL, Mackey MR, Murphy AN, Koller A, Perkins GA, Ellisman MH, Taylor SS (2011) ChChd3, an inner mitochondrial membrane protein, is essential for maintaining crista integrity and mitochondrial function. J Biol Chem 286(4):2918–2932. doi:10.1074/jbc.M110.171975
Dobson-Stone C, Shaw AD, Hallupp M, Bartley L, McCann H, Brooks WS, Loy CT, Schofield PR et al (2015) Is CHCHD10 Pro34Ser pathogenic for frontotemporal dementia and amyotrophic lateral sclerosis? Brain 138:e385. doi:10.1093/brain/awv115
Marroquin N, Stranz S, Muller K, Wieland T, Ruf WP, Brockmann SJ, Danzer KM, Borck G, et al. (2015) Screening for CHCHD10 mutations in a large cohort of sporadic ALS patients: no evidence for pathogenicity of the p.P34S variant. Brain. awv218. doi:10.1093/brain/awv218
Ronchi D, Riboldi G, Del Bo R, Ticozzi N, Scarlato M, Galimberti D, Corti S, Silani V et al (2015) CHCHD10 mutations in Italian patients with sporadic amyotrophic lateral sclerosis: figure 1. Brain 138(8):e372–e372. doi:10.1093/brain/awu384
Genin EC, Plutino M, Bannwarth S, Villa E, Cisneros-Barroso E, Roy M, Ortega-Vila B, Fragaki K et al (2015) CHCHD10 mutations promote loss of mitochondrial cristae junctions with impaired mitochondrial genome maintenance and inhibition of apoptosis. EMBO Mol Med 8(1):58–72. doi:10.15252/emmm.201505496
Penttilä S, Jokela M, Bouquin H, Saukkonen AM, Toivanen J, Udd B (2015) Late onset spinal motor neuronopathy is caused by mutation in CHCHD10. Ann Neurol 77(1):163–172. doi:10.1002/ana.24319
Pasanen P, Myllykangas L, Poyhonen M, Kiuru-Enari S, Tienari PJ, Laaksovirta H, Toppila J, Ylikallio E et al (2015) Intrafamilial clinical variability in individuals carrying the CHCHD10 mutation Gly66Val. Acta Neurol Scand. doi:10.1111/ane.12470
Wong CH, Topp S, Gkazi AS, Troakes C, Miller JW, de Majo M, Kirby J, Shaw PJ et al (2015) The CHCHD10 P34S variant is not associated with ALS in a UK cohort of familial and sporadic patients. Neurobiol Aging 36(10):2908 e2917–2908. doi:10.1016/j.neurobiolaging.2015.07.014
Acknowledgments
The authors appreciate all cohort individuals and their families for their participation in this study. The present study was supported by the funding of the National Science Fund of China (grant no. 81371394 and no.81511140101) and the Science and Technology Bureau Fund of Sichuan Province (2014FZ0072).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All participants were informed before enrolment and given a written informed consent form for the study under a protocol approved by the Ethics Committee of West China Hospital of Sichuan University.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
QingQing Zhou and YongPing Chen contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOCX 15 kb)
Supplementary Table 2
(DOCX 15 kb)
Supplementary Table 3
(DOCX 16 kb)
Supplementary Table 4
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Zhou, Q., Chen, Y., Wei, Q. et al. Mutation Screening of the CHCHD10 Gene in Chinese Patients with Amyotrophic Lateral Sclerosis. Mol Neurobiol 54, 3189–3194 (2017). https://doi.org/10.1007/s12035-016-9888-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9888-0