Abstract
The authors describe an electrochemiluminescence (ECL) based aptasensor for the pesticide aldicarb. The method is based on effective ECL energy transfer that occurs between the ruthenium(II) bipyridyl complex [referred to as Ru(bpy)3 2+] and gold nanoparticles (AuNPs). More specifically, multiwalled carbon nanotubes were modified with dendritic poly(L-arginine) labeled with Ru(bpy)3 2+, and the aptamers were taggedd with AuNPs. In the absence of aldicarb, the ECL emitted by Ru(bpy)3 2+ is enhanced by AuNPs under peak wavelength at at a wavelength of 610 nm. In the presence of aldicarb, the capture and competitive binding of aldicarb to the DNA aptamers causes their separation from the DPA6/Ru(bpy)3 2+/MWCNT. As a result, ECL intensity decreases linearly with increasing aldicarb concentrations in the range between 40 pM and 4 nM, with a detection limit of 9.6 pM. This aptamer switch is highly sensitive, selective and inexpensive. Conceivably, it can be adapted to formats for the determination of other pesticide residues by using different DNA aptamers.

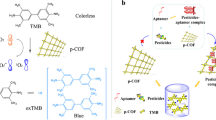
Schematic of the procedure for aptamer-based detection of aldicarb using the ECL signal of the Ru(bpy)3 2+ amplified by gold nanoparticles. This assay has high sensitivity, good selectivity, and low cost. It can presumably be transferred to other pesticide detection schemes.






Similar content being viewed by others
References
Miles CJ, Delfino JJ (1985) Fate of aldicarb, aldicarb sulfoxide, and aldicarb sulfone in Floridan groundwater. J Agr Food Chem 33:455–460
López-Fernández O, Rial-Otero R, Simal-Gándara J (2015) High-throughput HPLC-MS/MS determination of the persistence of neonicotinoid insecticide residues of regulatory interest in dietary bee pollen. Anal Bioanal Chem 407:7101–7110
Chow CF, Ho KYF, Gong CB (2015) Synthesis of a new bimetallic Re (I)-NCS-Pt (II) complex as chemodosimetric ensemble for the selective detection of mercapto-containing pesticides. Anal Chem 87:6112–6118
Liu F, Nordin AN, Li F, Voiculescu I (2014) A lab-on-chip cell-based biosensor for label-free sensing of water toxicants. Lab Chip 4:11270–11280
Hou T, Zhang L, Sun X, Li F (2016) Biphasic photoelectrochemical sensing strategy based on in situ formation of CdS quantum dots for highly sensitive detection of acetylcholinesterase activity and inhibition. Biosens Bioelectron 75:359–364
Baldrich E, Restrepo A, O'Sullivan CK (2004) Aptasensor development: elucidation of critical parameters for optimal aptamer performance. Anal Chem 76:7053–7063
Jia F, Duan N, Wu S, Dai R, Wang Z, Li X (2016) Impedimetric salmonella aptasensor using a glassy carbon electrode modified with an electrodeposited composite consisting of reduced graphene oxide and carbon nanotubes. Microchim Acta 18:337–344
Xu Y, Hun X, Liu F, Wen X, Luo X (2015a) Aptamer biosensor for dopamine based on a gold electrode modified with carbon nanoparticles and thionine labeled gold nanoparticles as probe. Microchim Acta 182:1797–1802
Chung E, Jeon J, Yu J, Lee C, Choo J (2015) Surface-enhanced raman scattering aptasensor for ultrasensitive trace analysis of bisphenol A. Biosens Bioelectron 64:560–565
Xu W, Xue S, Yi H, Jing P, Chai Y, Yuan R (2015b) A sensitive electrochemical aptasensor based on the co-catalysis of hemin/G-quadruplex, platinum nanoparticles and flower-like MnO2 nanosphere functionalized multi-walled carbon nanotubes. Chem Commun 51:1472–1474
Arduini F, Cinti S, Scognamiglio V, Moscone D (2016) Nanomaterials in electrochemical biosensors for pesticide detection: advances and challenges in food analysis. Microchim Acta 183:2063–2083
Ma K, Wang H, Li H, Wang S, Li X, Xu B, Tian W (2016) A label-free aptasensor for turn-on fluorescent detection of ATP based on AIE-active probe and water-soluble carbon nanotubes. Sensor Actuat B-Chem 230:556–558
Chen L, Zeng X, Ferhan AR, Chi Y, Kim DH, Chen G (2015) Signal-on electrochemiluminescent aptasensors based on target controlled permeable films. Chem Commun 51:1035–1038
Yao GH, Liang RP, Huang CF, Zhang L, Qiu JD (2015) Enzyme-free surface plasmon resonance aptasensor for amplified detection of adenosine via target-triggering strand displacement cycle and Au nanoparticles. Anal Chim Acta 871:28–34
Lei YM, Huang WX, Zhao M, Chai YQ, Yuan R, Zhuo Y (2015) Electrochemiluminescence resonance energy transfer system: mechanism and application in ratiometric aptasensor for lead ion. Anal Chem 87:7787–7794
Zhu S, Hong M, Tang G, Qian L, Lin J, Jiang Y, Pei Y (2010) Partly PEGylated polyamidoamine dendrimer for tumor-selective targeting of doxorubicin: the effects of PEGylation degree and drug conjugation style. Biomaterials 31:1360–1371
Kavosi B, Hallaj R, Teymourian H, Salimi A (2014) Au nanoparticles/PAMAM dendrimer functionalized wired ethyleneamine-viologen as highly efficient interface for ultra-sensitive α-fetoprotein electrochemical immunosensor. Biosens Bioelectron 59:389–396
Jiang X, Wang H, Yuan R, Chai Y (2015) Sensitive electrochemiluminescence detection for CA15–3 based on immobilizing luminol on dendrimer functionalized ZnO nanorods. Biosens Bioelectron 6333–38
Villalonga R, Díez P, Gamella M, Reviejo AJ, Romano S, Pingarrón JM (2012) Layer-by-layer supramolecular architecture of cyclodextrin-modified PAMAM dendrimers and adamantane-modified peroxidase on gold surface for electrochemical biosensing. Electrochim Acta 76:249–255
Liu L, Jiang S, Wang L, Zhang Z, Xie G (2015) Direct detection of micro RNA-126 at a femtomolar level using a glassy carbon electrode modified with chitosan, graphene sheets, and a poly (amidoamine) dendrimer composite with gold and silver nanoclusters. Microchim Acta 182:77–84
Fu C, Xu Q, Wei X, Li J (2014) Highly sensitive ECL immunosensor based on multi-labeling of luminol via a dendrimer on Fe3O4 nanoparticles. RSC Adv 4:26102–26107
Okuda T, Kawakami S, Maeie T, Niidome T, Yamashita F, Hashida M (2006) Biodistribution characteristics of amino acid dendrimers and their PEGylated derivatives after intravenous administration. J Control Release 114:69–77
Fields CG, Lloyd DH, Macdonald RL, Otteson KM, Noble RL (1991) BTU activation for automated fmoc solid phase peptide synthesis. Pept Res 4:95–101
Wang J, Shan Y, Zhao WW, Xu JJ, Chen HY (2011) Gold nanoparticle enhanced electrochemiluminescence of CdS thin films for ultrasensitive thrombin detection. Anal Chem 83:4004–4011
Shan Y, Xu JJ, Chen HY (2009) Distance-dependent quenching and enhancing of electrochemiluminescence from a CdS: Mn nanocrystal film by Au nanoparticles for highly sensitive detection of DNA. Chem Commun 8:905–907
Zong WL, Qu SX, Lu G (2013) Determination of aldicarb in ginger by HPLC-MS/MS. J Mater 2:63–67
Suprun E, Evtugyn G, Budnikov H, Ricci F, Moscone D, Palleschi G (2005) Acetylcholinesterase sensor based on screen-printed carbon electrode modified with prussian blue. Anal Bioanal Chem 383:597–604
Kok FN, Bozoglu F, Hasirci V (2002) Construction of an acetylcholinesterase-choline oxidase biosensor for aldicarb determination. Biosens Bioelectron 17:531–539
Azab HA, Duerkop A, Mogahed EM, Awad FK, Aal RM, Kamel RM (2012) Fluorescence and electrochemical sensing of pesticides methomyl, aldicarb and prometryne by the luminescent europium-3-carboxycoumarin probe. J Fluoresc 22:659–676
Evtugyn GA, Shamagsumova RV, Padnya PV, Stoikov II, Antipin IS (2014) Cholinesterase sensor based on glassy carbon electrode modified with ag nanoparticles decorated with macrocyclic ligands. Talanta 127:9–17
Acknowledgements
The authors gratefully acknowledge the financial support received from the Fundamental Scientific Research Funds for Chinese Academy of Tropical Agricultural Sciences (No. 1630042015011 and 1630042015009) and the Science and Technology Project of Hainan Province (No. ZDXM2015044).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 897 kb)
Rights and permissions
About this article
Cite this article
Li, S., Liu, C., Han, B. et al. An electrochemiluminescence aptasensor switch for aldicarb recognition via ruthenium complex-modified dendrimers on multiwalled carbon nanotubes. Microchim Acta 184, 1669–1675 (2017). https://doi.org/10.1007/s00604-017-2177-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2177-4