Abstract
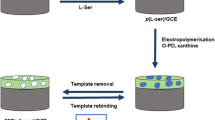
The authors describe the construction of a molecularly imprinted electrochemical sensor for enantiomeric recognition of L-phenylalanine (L-Phe). Firstly, thiolated β-cyclodextrin (β-CD) and L-cysteine were used to functionalize gold nanoparticles via gold-thiol chemistry and to act as a basis for the formation of a microporous metal-organic framework (MOF). A MOF imprinted polymer was deposited on the functionalized gold nanoparticles by electropolymerization in the presence of the template L-Phe and the functional monomer 4-aminothiophenol. The modified electrode was characterized by cyclic voltammetry and electrochemical impedance spectroscopy in the presence of hexacyanoferrate acting as the redox probe. The electrode, operated best at 0.2 V vs. Ag/AgCl, exhibits excellent selectivity for L-Phe, with an enantioselectivity coefficient of 2.12 over D-Phe. In addition to its good enantiomeric selectivity, the sensor has a 0.33 pM detection limit which is much lower than that reported for other electrodes. The sensor has been successfully applied to the analysis of urine spiked with L-Phe.

Schematic of a microporous metal organic framework imprinting sensor for the analysis of enantiomers. Combined with MOF and β-CD, the sensor exhibits excellent selectivity and sensitivity for chiral assay of L-phenylalanine.







Similar content being viewed by others
References
Wei Y, Tian AL, Li Y, Wang X, Cao B (2012) A general chiral selector immobilized on silica magnetic microspheres for direct separation of racemates. J Mater Chem 22:8499–8504. doi:10.1039/C2JM30372H
Bogunovic L, Fliedner M, Eichhorn R, Wegener S, Regtmeier J, Anselmetti D, Reimann P (2012) Chiral particle separation by a nonchiral microlattice. Phys Rev Lett 109:2523–2527. doi:10.1103/PhysRevLett.109.100603
Fireman-shoresh S, Turyan I, Mandler D, Avnir D, Marx S (2005) Chiral electrochemical recognition by very thin molecularly imprinted sol-gel films. Langmuir 21:7842–7847. doi:10.1021/la050240y
Maier NM, Lindner W (2007) Chiral recognition applications of molecularly imprinted polymers: a critical review. Anal Bioanal Chem 389:377–397. doi:10.1007/s00216-007-1427-4
Fu Q, Sanbe H, Kagawa C, Kunimoto KK, Haginaka J (2003) Uniformly sized molecularly imprinted polymer for (S)-Nilvadipine. Comparison of chiral recognition ability with HPLC chiral stationary phases based on a protein. Anal Chem 75:191–198. doi:10.1021/ac026039z
Jiang Z, Yu Y, Wu H (2006) Preparation of CS/GPTMS hybrid molecularly imprinted membrane for efficient chiral resolution of phenylalanine isomers. J Membrane Sci 280:876–882. doi:10.1016/j.memsci.2006.03.006
Niu M, Chuong P-H, He H (2016) Core-shell nanoparticles coated with molecularly imprinted polymers: a review. Microchim Acta 183:2677–2695. doi:10.1007/s00604-016-1930-4
Guo ZZ, Florea A, Cristea C, Bessueille F, Vocanson F, Goutaland F, Zhang AD, Săndulescu R, Lagarde F, Jaffrezic-Renault N (2015) 1,3,5-trinitrotoluene detection by a molecularly imprinted polymer sensor based on electropolymerization of a microporous-metal-organic framework. Sensor Actuat B-chem 207:960–966. doi:10.1016/j.snb.2014.06.137
Florea A, Guo ZZ, Cristea C, Bessueille F, Vocanson F, Goutaland F, Dzyadeyych S, Sandulescu R, Jaffrezic-Renault N (2015) Anticancer drug detection using a highly sensitive molecularly imprinted electrochemical sensor based on an electropolymerized microporous metal organic framework. Talanta 138:71–76. doi:10.1016/j.talanta.2015.01.013
Riskin M, Telvered R, Frasconi M, Yavo N, Willner I (2010) Stereoselective and chiroselective surface plasmon resonance (SPR) analysis of amino acids by molecularly imprinted Au-nanoparticle composites. Chem-Eur J 16(24):7114–7120. doi:10.1002/chem.200903215
Liu HM, Liu CH, Yang XJ, Zeng SJ, Xiong Y, Xu W (2008) Uniformly sized β-cyclodextrin molecularly imprinted microspheres prepared by a novel surface imprinting technique for ursolic acid. Anal Chim Acta 628:87–94. doi:10.1016/j.aca.2008.08.042
Kyzas GZ, Lazaridis NK, Bikiaris DN (2013) Optimization of chitosan and β-cyclodextrin molecularly imprinted polymer synthesis for dye adsorption. Carbohyd Polym 91:198–208. doi:10.1016/j.carbpol.2012.08.016
Glushakov AV, Dennis DM, Morey TE, Sumners C, Cucchiara RF, Seubert CN, Martynyuk AE (2002) Specific inhibition of N-methyl-D-aspartate receptor function in rat hippocampal neurons by L-phenylalanine at concentrations observed during phenylketonuria. Mol Psychiatry 7:359–367. doi:10.1038/sj/mp/4000976
Martynyuk AE, Glushakov AV, Sumners C, Laipis PJ, Dennis DM, Seubert CN (2005) Impaired glutamatergic synaptic transmission in the PKU brain. Mol Genet Metab 86:34–42. doi:10.1016/j.ymgme.2005.06.014
Parra MJA, Mateos AA, Mata MM, María CG (1998) Enzymatic flow-injection determination of L-phenylalanine using the stopped-flow and merging-zones techniques. Talanta 47:121–126
Bi PY, Dong HR, Yu HB, Chang L (2008) Separation and enrichment of L-phenylalanine by flotation complexation extraction. Chinese J Anal Chem 36:658–662
Wei YL, Li HH, Hao HY, Chen YX, Dong C, Wang GF (2014) β-Cyclodextrin functionalized Mn-doped ZnS quantum dots for the chiral sensing of tryptophan enantiomers. Polym Chem 6:591–598. doi:10.1039/c4py00618f
Zhang ZX, Zhou J, Liu Y, Tang J, Tang WH (2015) Cyclodextrin capped CdTe quantum dots as versatile fluorescence sensors for nitrophenol isomers. Nano 7:19540–19546. doi:10.1039/c5nr06073g
Tang J, Zhang LL, Liu Y, Zhou J, Han GQ, Tang WH (2014) Gold nanoparticles-β-cyclodextrin-chitosan-gaphene modified glassy carbon electrode for ultrasensitive detection of dopamine and uric acid. Electroanalysis 26:2057–2064. doi:10.1002/elan.201400272
Li JP, Sun M, Wei XP, Zhang LP, Zhang Y (2015) An electrochemical aptamer biosensor based on “gate-controlled” effect using β-cyclodextrin for ultra-sensitive detection of trace mercury. Biosens Bioelectron 74:423–426. doi:10.1016/j.bios.2015.06.061
Wang YX, Yin XL, Shi MH, Li W, Zhang L, Kong JL (2006) Probing chiral amino acids at sub-picomolar level based on bovine serum albumin enantioselective films coupled with silver-enhanced gold nanoparticles. Talanta 69:1240–1245. doi:10.1016/j.talanta.2005.12.060
Lian WJ, Huang JD, Yu JH, Zhang XM, Lin Q, He XR, Xing XR, Liu S (2012) A molecularly imprinted sensor based on β-cyclodextrin incorporated multiwalled carbon nanotube and gold nanoparticles-polyamide amine dendrimer nanocomposites combining with water-soluble chitosan derivative for the detection of chlortetracycline. Food Control 26:620–627. doi:10.1016/j.foodcont.2012.02.023
Pan JM, Zou XH, Wang X, Guan W, Yan YS, Han J (2010) Selective recognition of 2,4-dichlorophenol from aqueous solution by uniformly sized molecularly imprinted microspheres with β-cyclodextrin/attapulgite composites as support. Chem Eng J 162:910–918. doi:10.1016/j.cej.2010.06.039
Acknowledgements
The authors gratefully acknowledge the financial support from National Natural Science Foundation of China (No. 21375031), the Natural Science Foundation of Guangxi Province of China (No. 2015GXNSFAA139029, 2015GXNSFFA139005), Guangxi Colleges and Universities Key Laboratory of Food Safety and Detection, High Level Innovation Teams of Guangxi Colleges & Universities and Outstanding Scholars Program (Guijiaoren[2014] 49) for this research project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 1.29 mb)
Rights and permissions
About this article
Cite this article
Wu, T., Wei, X., Ma, X. et al. Amperometric sensing of L-phenylalanine using a gold electrode modified with a metal organic framework, a molecularly imprinted polymer, and β-cyclodextrin-functionalized gold nanoparticles. Microchim Acta 184, 2901–2907 (2017). https://doi.org/10.1007/s00604-017-2281-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2281-5