Abstract
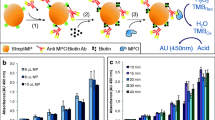
This research describes a nanowire network-based method for detecting the activity of myeloperoxidase (MPO), a biomarker of acute coronary syndromes (ACS). Trimetallic CuPdPt nanowire networks (CuPdPt NWNWs) were prepared by a one-step chemical reduction method. The metallic precursors quickly form nanowire network structures without the need for additional capping agents or surfactants. This process creates a product with a clean surface. The NWNWs were dropped onto a glassy carbon electrode (GCE) to obtain a sensor with good catalytic activity towards the reduction of hydrogen peroxide (H2O2), which was used as an electrochemical probe working at −0.4 V (vs. SCE). It also provided a large surface for further modification. Next, an antibody against MPO was immobilized on the modified GCE via the stable conjunction between Cu, Pt, Pd and amino groups. Upon binding of MPO to the antibody on the GCE, the current response to H2O2 was reduced by 35 μA·cm−2. The immunosensor had a linear response within the 100 fg·mL−1 to 50 ng·mL−1 MPO concentration range and a 33 fg·mL−1 detection limit (at an S/N ratio of 3). The recovery of spiked serum samples ranged from 99.8 to 103.6%. This result suggests that the method can be applied to the quantitation of MPO in human serum samples.

A trimetallic CuPdPt nanowire networks was placed on a glassy cabon electrode (GCE) to design an immunosensor for myeloperoxidase (MPO), a biomarker for the acute coronary syndrome (ACS). Antibody against MPO was immobilized on the network via conjugation between Cu, Pt, Pd and amino groups. Amperometric i-t measurements were conducted to quantify the amount of MPO that binds to the antibody on the surface of the modified GCE.






Similar content being viewed by others
References
Nicholls SJ, Hazen SL (2005) Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 25:1102–1111. https://doi.org/10.1161/01.ATV.0000163262.83456.6d
Frossard M, Fuchs I, Leitner JM et al (2004) Platelet function predicts myocardial damage in patients with acute myocardial infarction. Circulation 110:1392–1397. https://doi.org/10.1161/01.CIR.0000141575.92958.9C
Mocatta TJ, Pilbrow AP, Cameron VA et al (2007) Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J Am Coll Cardiol 49:1993–2000. https://doi.org/10.1016/j.jacc.2007.02.040
Franck T, Grulke S, Deby-Dupont G et al (2005) Development of an enzyme-linked immunosorbent assay for specific equine neutrophil myeloperoxidase measurement in blood. J Vet Diagn Investig 17:412–419. https://doi.org/10.1177/104063870501700502
Gandley RE, Rohland J, Zhou Y et al (2008) Increased myeloperoxidase in the placenta and circulation of women with preeclampsia. Hypertension 52:387–393. https://doi.org/10.1161/HYPERTENSIONAHA.107.107532
Ghindilis AL, Atanasov P, Wilkins M et al (1998) Immunosensors: electrochemical sensing and other engineering approaches. Biosens Bioelectron 13:113–131
Wang X, Tao G, Meng Y (2009) Nanogold hollow microsphere-based electrochemical immunosensor for the detection of ferritin in human serum. Microchim Acta 167:147–152. https://doi.org/10.1007/s00604-009-0225-4
Kim DM, Noh HB, Park DS et al (2009) Immunosensors for detection of Annexin II and MUC5AC for early diagnosis of lung cancer. Biosens Bioelectron 25:456–462. https://doi.org/10.1016/j.bios.2009.08.007
Mani V, Chikkaveeraiah BV, Patel V et al (2009) Ultrasensitive immunosensor for cancer biomarker proteins using gold nanoparticle film electrodes and multienzyme-particle amplification. ACS Nano 3:585–594. https://doi.org/10.1021/nn800863w
Loyprasert S, Thavarungkul P, Asawatreratanakul P et al (2008) Label-free capacitive immunosensor for microcystin-LR using self-assembled thiourea monolayer incorporated with Ag nanoparticles on gold electrode. Biosens Bioelectron 24:78–86. https://doi.org/10.1016/j.bios.2008.03.016
Thavarungkul P, Dawan S, Kanatharana P et al (2007) Detecting penicillin G in milk with impedimetric label-free immunosensor. Biosens Bioelectron 23:688–694. https://doi.org/10.1016/j.bios.2007.08.003
Lu L, Liu B, Li S et al (2011) Improved electrochemical immunosensor for myeloperoxidase in human serum based on nanogold/cerium dioxide-BMIMPF6/L-cysteine composite film. Colloids Surf B: Biointerfaces 86:339–344. https://doi.org/10.1016/j.colsurfb.2011.04.017
Venkatraman VL, Reddy RK, Zhang F et al (2009) Iridium oxide nanomonitors: clinical diagnostic devices for health monitoring systems. Biosens Bioelectron 24:3078–3083. https://doi.org/10.1016/j.bios.2009.03.029
Moral-Vico J, Barallat J, Abad L et al (2015) Dual chronoamperometric detection of enzymatic biomarkers using magnetic beads and a low-cost flow cell. Biosens Bioelectron 69:328–336. https://doi.org/10.1016/j.bios.2015.02.042
Windmiller JR, Chinnapareddy S, Santhosh P et al (2010) Strip-based amperometric detection of myeloperoxidase. Biosens Bioelectron 26:886–889. https://doi.org/10.1016/j.bios.2010.07.031
Zou Z, Kai J, Rust MJ et al (2007) Functionalized nano interdigitated electrodes arrays on polymer with integrated microfluidics for direct bio-affinity sensing using impedimetric measurement. Sensors Actuators A Phys 136:518–526. https://doi.org/10.1016/j.sna.2006.12.006
Prasad S, Quijano J (2006) Development of nanostructured biomedical micro-drug testing device based on in situ cellular activity monitoring. Biosens Bioelectron 21:1219–1229. https://doi.org/10.1016/j.bios.2005.05.005
Zhang Y, Kolmakov A, Lilach Y et al (2005) Electronic control of chemistry and catalysis at the surface of an individual tin oxide nanowire. J Phys Chem B 109:1923–1929. https://doi.org/10.1021/jp045509l
Guo S, Li D, Zhu H et al (2013) FePt and CoPt nanowires as efficient catalysts for the oxygen reduction reaction. Angew Chem Int Ed Eng 52:3465–3468. https://doi.org/10.1002/anie.201209871
Guo S, Zhang S, Sun X et al (2011) Synthesis of ultrathin FePtPd nanowires and their use as catalysts for methanol oxidation reaction. J Am Chem Soc 133:15354–15357. https://doi.org/10.1021/ja207308b
Liu R, Liu JF, Jiang GB (2010) Use of triton X-114 as a weak capping agent for one-pot aqueous phase synthesis of ultrathin noble metal nanowires and a primary study of their electrocatalytic activity. Chem Commun (Camb) 46:7010–7012. https://doi.org/10.1039/c0cc02466j
Saleem F, Zhang Z, Xu B et al (2013) Ultrathin Pt-cu nanosheets and nanocones. J Am Chem Soc 135:18304–18307. https://doi.org/10.1021/ja4101968
Xu D, Liu Z, Yang H et al (2009) Solution-based evolution and enhanced methanol oxidation activity of monodisperse platinum-copper nanocubes. Angew Chem Int Ed Eng 48:4217–4221. https://doi.org/10.1002/anie.200900293
Yin AX, Min XQ, Zhu W et al (2012) Pt-cu and Pt-Pd-cu concave nanocubes with high-index facets and superior electrocatalytic activity. Chemistry 18:777–782. https://doi.org/10.1002/chem.201102632
Hong W, Wang J, Wang E (2016) Scalable synthesis of cu-based ultrathin nanowire networks and their electrocatalytic properties. Nano 8:4927–4932. https://doi.org/10.1039/c5nr07516e
Wu D, Fan H, Li Y et al (2013) Ultrasensitive electrochemical immunoassay for squamous cell carcinoma antigen using dumbbell-like Pt-Fe(3)O(4) nanoparticles as signal amplification. Biosens Bioelectron 46:91–96. https://doi.org/10.1016/j.bios.2013.02.014
Chen KJ, Lee CF, Rick J et al (2012) Fabrication and application of amperometric glucose biosensor based on a novel PtPd bimetallic nanoparticle decorated multi-walled carbon nanotube catalyst. Biosens Bioelectron 33:75–81. https://doi.org/10.1016/j.bios.2011.12.020
Mu Y, Liang H, Hu J et al (2005) Controllable pt nanoparticle deposition on carbon nanotubes as an anode catalyst for direct methanol fuel cells. J Phys Chem B 109:22212–22216. https://doi.org/10.1021/jp0555448
Wang J (2005) Nanomaterial-based electrochemical biosensors. Analyst 130:421–426. https://doi.org/10.1039/B414248A
Gao F, Goodman DW (2012) Pd-Au bimetallic catalysts: understanding alloy effects from planar models and (supported) nanoparticles. Chem Soc Rev 41:8009–8020. https://doi.org/10.1039/c2cs35160a
Tian L, Liu L, Li Y et al (2016) A novel label-free electrochemical immunosensor for the detection of hepatitis B surface antigen. Anal Methods 8:7380–7386. https://doi.org/10.1039/c6ay01959e
Zhu H, Zhang S, Guo S et al (2013) Synthetic control of FePtM nanorods (M = Cu, Ni) to enhance the oxygen reduction reaction. J Am Chem Soc 135:7130–7133. https://doi.org/10.1021/ja403041g
Choi SI, Xie S, Shao M et al (2013) Synthesis and characterization of 9 nm Pt-Ni octahedra with a record high activity of 3.3 A/mg(Pt) for the oxygen reduction reaction. Nano Lett 13:3420–3425. https://doi.org/10.1021/nl401881z
Yang H, Zhang J, Sun K et al (2010) Enhancing by weakening: electrooxidation of methanol on Pt3Co and Pt nanocubes. Angew Chem Int Ed Eng 49:6848–6851. https://doi.org/10.1002/anie.201002888
Mazumder V, Chi M, Mankin MN et al (2012) A facile synthesis of MPd (M = Co, Cu) nanoparticles and their catalysis for formic acid oxidation. Nano Lett 12:1102–1106. https://doi.org/10.1021/nl2045588
Acknowledgments
We are grateful for the financial support from the National Nature Science Foundation of China (No. 81370403), the Chongqing Foundation and Advanced Research Project (No. CSTC2015jcyjBX0053), the Chongqing Precision Medical Key Technology Research and Development and Demonstration Projects (cstc2016shms-ztzx0042) and the Chongqing Medical University Scientific Research Cultivating Fund (No.201414).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 439 kb)
Rights and permissions
About this article
Cite this article
Wen, Y., Yuan, J., Chen, J. et al. Amperometric myeloperoxidase immunoassay based on the use of CuPdPt nanowire networks. Microchim Acta 185, 55 (2018). https://doi.org/10.1007/s00604-017-2563-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2563-y