Abstract
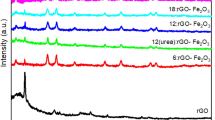
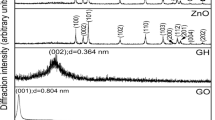
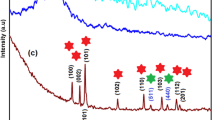
High-quality Prussian blue/reduced graphene oxide (PB/rGO) nanohybrids were synthesized via a simple polyvinylpyrrolidone (PVP)-assisted polyol reduction method under mild conditions. The structure and composition of PB/rGO were confirmed by means of X-ray diffraction (XRD), electron microscopes (SEM and transmission electron microscopy (TEM)), Fourier transform infrared (FTIR), and X-ray photoelectron spectroscopy (XPS). These results indicate that ratios of starting materials allow a good control on loading and morphology of PB/graphene hybrids, and at ratio of K3Fe(CN)6/GO of 1:2, PB nanocubes get completely embedded into the defect of porous graphene matrices. Electrochemcial characterization of the PB/rGO nanocomposites with different PB/rGO weight ratios was carried out by cyclic voltammograms and galvanostatic charge–discharge in 1.0 M KNO3 electrolyte. The PB/rGO nanocomposites exhibit much higher specific capacitances than either bare PB nanocrystals or pure rGO sheets. PB/rGO (1:2) exhibits the highest specific capacitance of 251.6 F g−1 at a scan rate of 10 mV s−1 and an excellent cycling stability along with 92 % specific capacitance retained after 1000 cycle tests. The significant enhancement in electrochemical performance over PB/rGO nanocomposites can be attributed to a positive synergetic effect that the PB nanocube interlocked dispersion of rGO sheets superimposes pseudocapacitance from PB on double-layer capacitance from rGO sheets.







Similar content being viewed by others
References
Conway BE (1999) Electrochemical supercapacitors. Scientific fundamentals and technological applications. Kluwer Academic Publishers Plenum Press, New York
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41:797–828
Inagaki M, Konno H, Tanaike O (2010) Carbon materials for electrochemical capacitors. J Power Sources 195:7880–7903
Frackowiak E (2007) Carbon materials for supercapacitor application. Phys Chem Chem Phys 9:1774–1785
Lei Z, Christov N, Zhao XS (2011) Intercalation of mesoporous carbon spheres between reduced graphene oxide sheets for preparing high-rate supercapacitor electrodes. Energy Environ Sci 4:1866–1873
Ma X, Liu M, Gan L, Zhao Y, Chen L (2013) Synthesis of micro- and mesoporous carbon spheres for supercapacitor electrode. J Solid State Electrochem 17:2293–2301
Zhibin L, Christov N, Li Li Z, Zhao XS (2011) Mesoporous carbon nanospheres with an excellent electrocapacitive performance. J Mater Chem 21:2274–2281
Zhibin L, Shiying B, Yi X, Liqin D, Lizhen A, Guangning Z, Qian X (2008) CMK-5 mesoporous carbon synthesized via chemical vapor deposition of ferrocene as catalyst support for methanol oxidation. J Phys Chem C 112:722–731
Gogotsi Y, Nikitin A, Ye HH, Zhou W, Fischer JE, Bo Y, Foley HC, Barsoum MW (2003) Nanoporous carbide-derived carbon with tunable pore size. Nat Mater 2:591–594
Tsai W-Y, Gao P-C, Daffos B, Taberna P-L, Perez CR, Gogotsi Y, Favier F, Simon P (2013) Ordered mesoporous silicon carbide-derived carbon for high-power supercapacitors. Electrochem Commun 34:109–112
Wang X, Liu L, Wang X, Bai L, Wu H, Zhang X, Yi L, Chen Q (2011) Preparation and performances of carbon aerogel microspheres for the application of supercapacitor. J Solid State Electrochem 15:643–648
Lazzari M, Soavi F, Mastragostino M (2008) High voltage, asymmetric EDLCs based on xerogel carbon and hydrophobic IL electrolytes. J Power Sources 178:490–496
Niu CM, Sichel EK, Hoch R, Moy D, Tennent H (1997) High power electrochemical capacitors based on carbon nanotube electrodes. Appl Phys Lett 70:1480–1482
Sun YQ, Wu QO, Shi GQ (2011) Graphene based new energy materials. Energy Environ Sci 4:1113–1132
Vivekchand S, Rout C, Subrahmanyam K, Govindaraj A, Rao CRN (2008) Graphene-based electrochemical supercapacitors. J Chem Sci 120:9–13
Pumera M (2011) Graphene-based nanomaterials for energy storage. Energy Environ Sci 4:668–674
Vangari M, Pryor T, Jiang L (2013) Supercapacitors: review of materials and fabrication methods. J Energy Eng ASCE 139:72–79
Chen J, Huang K, Liu S, Hu X (2009) Electrochemical supercapacitor behavior of Ni3(Fe(CN)6)2(H2O) nanoparticles. J Power Sources 186:565–569
Chen J, Huang K, Liu S (2008) Insoluble metal hexacyanoferrates as supercapacitor electrodes. Electrochem Commun 10:1851–1855
Lisowska-Oleksiak A, Nowak AP (2007) Metal hexacyanoferrate network synthesized inside polymer matrix for electrochemical capacitors. J Power Sources 173:829–836
Lu XJ, Dou H, Gao B, Yuan CZ, Yang SD, Hao L, Shen LF, Zhang XG (2011) A flexible graphene/multiwalled carbon nanotube film as a high performance electrode material for supercapacitors. Electrochim Acta 56:5115–5121
Wang H, Hao Q, Yang X, Lu L, Wang X (2010) A nanostructured graphene/polyaniline hybrid material for supercapacitors. Nanoscale 2:2164–2170
Wilde RE, Ghosh SN, Marshall BJ (1970) Prussian blues. Inorg Chem 9:2512–2516
Jin E, Lu X, Cui L, Chao D, Wang C (2010) Fabrication of graphene/prussian blue composite nanosheets and their electrocatalytic reduction of H2O2. Electrochim Acta 55:7230–7234
Jiang YY, Zhang XD, Shan CS, Hua SC, Zhang QX, Bai XX, Dan L, Niu L (2011) Functionalization of graphene with electrodeposited Prussian blue towards amperometric sensing application. Talanta 85:76–81
Cao L, Liu Y, Zhang B, Lu L (2010) In situ controllable growth of Prussian blue nanocubes on reduced graphene oxide: facile synthesis and their application as enhanced nanoelectrocatalyst for H2O2 reduction. ACS Appl Mater Interfaces 2:2339–2346
Mao Y, Bao Y, Wang W, Li Z, Li F, Niu L (2011) Layer-by-layer assembled multilayer of graphene/Prussian blue toward simultaneous electrochemical and SPR detection of H2O2. Talanta 85:2106–2112
Zhang Y, Sun XM, Zhu LZ, Shen HB, Jia NQ (2011) Electrochemical sensing based on graphene oxide/Prussian blue hybrid film modified electrode. Electrochim Acta 56:1239–1245
Zhao G, Feng J-J, Zhang Q-L, Li S-P, Chen H-Y (2005) Synthesis and characterization of Prussian blue modified magnetite nanoparticles and its application to the electrocatalytic reduction of H2O2. Chem Mater 17:3154–3159
Qiu J-D, Peng H-Z, Liang R-P, Li J, Xia X-H (2007) Synthesis, characterization, and immobilization of Prussian blue-modified Au nanoparticles: application to electrocatalytic reduction of H2O2. Langmuir 23:2133–2137
Chen D, Feng H, Li J (2012) Graphene oxide: preparation, functionalization, and electrochemical applications. Chem Rev 112:6027–6053
Prakash A, Chandra S, Bahadur D (2012) Structural, magnetic, and textural properties of iron oxide-reduced graphene oxide hybrids and their use for the electrochemical detection of chromium. Carbon 50:4209–4219
Wang S, Jiang SP, Wang X (2011) Microwave-assisted one-pot synthesis of metal/metal oxide nanoparticles on graphene and their electrochemical applications. Electrochim Acta 56:3338–3344
Bonanni A, Ambrosi A, Pumera M (2012) On oxygen-containing groups in chemically modified graphenes. Chem Eur J 18:4541–4548
Shen X, Wu S, Liu Y, Wang K, Xu Z, Liu W (2009) Morphology syntheses and properties of well-defined Prussian blue nanocrystals by a facile solution approach. J Colloid Interface Sci 329:188–195
Liu Q, Zhu X, Huo Z, He X, Liang Y, Xu M (2012) Electrochemical detection of dopamine in the presence of ascorbic acid using PVP/graphene modified electrodes. Talanta 97:557–562
Washio I, Xiong Y, Yin Y, Xia Y (2006) Reduction by the end groups of poly(vinyl pyrrolidone): a new and versatile route to the kinetically controlled synthesis of Ag triangular nanoplates. Adv Mater 18:1745–1749
Ghosh D, Giri S, Mandal M et al (2014) High performance supercapacitor electrode material based on vertically aligned PANI grown on reduced graphene oxide/Ni(OH)2 hybrid composite. RSC Adv 4(50):26094–26101
Acknowledgments
This work has been sponsored by the National Natural Science Foundation of China (No. 21361020, 21361019), Enhance Comprehensive Strength Project of Ningxia University (8016-18), National Undergraduate Innovation Program of China (131074901), Project of State Key Laboratory of Catalysis, and Dalian Institute of Chemical Physics of The Chinese Academy of Sciences (N-09-13).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, M., Dou, Y., Kang, H. et al. A novel interlocked Prussian blue/reduced graphene oxide nanocomposites as high-performance supercapacitor electrodes. J Solid State Electrochem 19, 1621–1631 (2015). https://doi.org/10.1007/s10008-015-2785-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-2785-z