Abstract
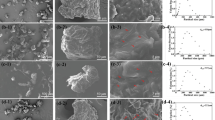
At the submarine screen doors of ships, the flow of water was fast and organic coatings were easy to peel off. To solve the problems of biofouling, the Cu/Cu2O coatings were prepared by cold spray technology. In this paper, the release mechanism of effective antifouling components of the coatings was studied by micro-domain electrochemical potential scanning technique. Cuprous oxide in the coatings and the surrounding copper constituted micro-cells which accelerated the local electrochemical dissolution of copper. Cuprous oxide acted as the cathode, and the surrounding copper acted as the anode. Meanwhile, under the action of current, chloride ions were transferred from cathode to the anode and promoted local electrochemical dissolution of copper. Experiments show that the higher the Cu2O content in the coatings, the greater the release rate of dissolved copper and inhibitory effect on diatoms. In the environments varied in dissolved oxygen, salinity, temperature and flow rate, the coatings maintained sufficient release rate of dissolved copper, unless the salinity was very low. Most of the dissolved copper was provided by the electrochemical dissolution process of copper. Whether in the indoor test or marine environment experiments, 30% Cu2O coatings show the best performance. After insulating layer was sprayed, the effect of antifouling was improved evidently and the coatings were applied to the submarine screen doors of ships.
















Similar content being viewed by others
References
I. Davidson, C. Scianni, C. Hewitt, R. Everett, E. Holm, M. Tamburri, and G. Ruiz, Mini-Review: Assessing the Drivers of Ship Biofouling Management—Aligning Industry and Biosecurity Goals, Biofouling, 2016, 32(4), p 411–428
Y.K. Demirel, D. Uzun, Y. Zhang, H.-C. Fang, A.H. Day, and O. Turan, Effect of Barnacle Fouling on Ship Resistance and Powering, Biofouling, 2017, 33, p 1–16
J. Monty, E. Dogan, R. Hanson, A. Scardino, B. Ganapathisubramani, and N. Hutchins, An Assessment of the Ship Drag Penalty Arising from Light Calcareous Tubeworm Fouling, Biofouling, 2016, 32(4), p 451–464
X. Li, G. Zhu, L. Dong, C. Ni, X. Yan, and L. Yu, Synthesis, Crystal Structure, and Theoretical Calculation of the Cu(II) Complex With 1,2-Benzisothiazolin-3-One, Synth. React. Inorg. Met. Org. Chem., 2016, 46(5), p 659–664
G. Zhu, L. Yu, X. Li, S. Xia, and X. Yan, Synthesis, Crystal Structure, and Theoretical Calculation of the Cu (II) Complex with 2-Furoic Acid, Synth. React. Inorg. Met. Org. Chem., 2014, 44(7), p 1054–1058
Y. Liu, X. Suo, Z. Wang, Y. Gong, X. Wang, and H. Li, Developing Polyimide-Copper Antifouling Coatings with Capsule Structures for Sustainable Release of Copper, Mater. Des., 2017, 130, p 285–293
Y. Xiong, G. Wang, Z. Xie, Y. Li, A. Wang, and H. Jiang, Synthesis of Cu2O Hollow Microspheres and Its Application in Antifouling Materials, Paint Ind., 2017, 47(4), p 55–65
H. Gao, L. Yu, J. Zhao, and J. Sui, Preparation of Nanometer Cuprous Oxide and Its Application in Antifouling Coatings, Shanghai Coat., 2008, 46(12), p 30–33
J. Sui, Study on Preparation of Ultrafine Cuprous Oxide and Its Modification, Ocean University of China, Qingdao Shi, 2005
V. Champagne and D. Helfritch, The Unique Abilities of Cold Spray Deposition, Int. Mater. Rev., 2016, 61(7), p 437–455
M. Rokni, S. Nutt, C. Widener, V. Champagne, and R. Hrabe, Review of Relationship Between Particle Deformation, Coating Microstructure, and Properties in High-Pressure Cold Spray, J. Therm. Spray Technol., 2017, 26(6), p 1308–1355
A. Sova, R. Maestracci, M. Jeandin, P. Bertrand, and I. Smurov, Kinetics of Composite Coating Formation Process in Cold Spray: Modelling And Experimental Validation, Surf. Coat. Technol., 2017, 318, p 309–314
D. Rui, L. Xiangbo, W. Jia, and X. Likun, Electrochemical Corrosion and Mathematical Model of Cold Spray Cu-Cu2O Coating in NaCl Solution—Part I: Tafel Polarization Region Model, Int. J. Electrochem. Sci., 2013, 8, p 5902–5924
D. Rui, H. Yu, and X. Li, Electrochemical Corrosion and Mathematical Model of Cold Spray Copper Composite Coating—Part II: Limiting Current Region, Int. J. Electrochem. Sci., 2017, 12(2), p 1232–1246
C. Stenson, K.A. Mcdonnell, S. Yin, B. Aldwell, M. Meyer, D.P. Dowling, and R. Lupoi, Cold Spray Deposition to Prevent Fouling of Polymer Surfaces, Surf. Eng., 2016, 1, p 1–11
R. Lupoi, C. Stenson, K.A. Mcdonnell, D.P. Dowling, and E. Ahearne, Antifouling Coatings Made with Cold Spray onto Polymers: Process Characterization, CIRP Ann. Manuf. Technol., 2016, 65(1), p 545–548
R. Ding, Study on Preparation, Anticorrosion and Antifouling Properties of Copper Composite Coatings Prepared by Cold Spray Technology, Ocean University of China, Qingdao Shi, 2014
Y. Hou, L. Xu, C. Shen, and X. Li, Electrochemical Characterization for Corrosion Resistance of Plasma-Sprayed Cr2O3 Coating with Sealing, J. Chin. Soc. Corros. Prot., 2012, 32(6), p 473–477
O. Andrews, E. Buitenhuis, C. Le Quéré, and P. Suntharalingam, Biogeochemical Modelling of Dissolved Oxygen in a Changing Ocean, Philos. Trans. R. Soc. A, 2017, 375(2102), p 20160328
A. Hameau, F. Joos, J. Mignot, K. Keller, Variability of dissolved oxygen over the last millennium and the 21st century in CESM, in EGU General Assembly Conference Abstracts (2017), p. 14031
L. Stramma, S. Schmidtko, M. Visbeck, Deoxygenation of the ocean, in Goldschmidt Conference (2017), p 13–18
M. Benallal, H. Moussa, F. Touratier, C. Goyet, and A. Poisson, Ocean Salinity from Satellite-Derived Temperature in the Antarctic Ocean, Antarct. Sci., 2016, 28(2), p 127–134
M. Munnich, A. Haumann, S. Eberenz, N. Gruber, Modeling the Influence of Land and Sea Ice on Southern Ocean Salinity and its Recent Trends, AGU Fall Meeting Abstracts (2016)
J. Scott, T. Meissner, F. Wentz, Ocean Surface Salinity from the SMAP Sensor, American Geophysical Union, Ocean Sciences Meeting 2016, abstract# PO44E-3212 (2016)
B. Bereiter, J. Severinghaus, S. Shackleton, D. Baggenstos, K. Kawamura, Mean ocean temperature change over the last glacial transition based on heavy noble gases in the atmosphere, in EGU General Assembly Conference Abstracts (2017), p. 5247
S.A. Keith, J.A. Maynard, A.J. Edwards, J.R. Guest, A.G. Bauman, R. Van Hooidonk, S.F. Heron, M.L. Berumen, J. Bouwmeester, S. Piromvaragorn, Coral mass spawning predicted by rapid seasonal rise in ocean temperature, in Proceedings of Royal Society B. The Royal Society (2016), p. 20160011
G.C. Hays, Ocean Currents and Marine Life, Curr. Biol., 2017, 27(11), p R470–R473
E. Wolbrecht, J. Osborn, S. Qualls, R. Ross, J. Canning, M. Anderson, D. Edwards, Estimating and compensating for water currents: field testing, in OCEANS 2016 MTS/IEEE Monterey (IEEE, 2016), pp. 1–5
H. Yanfeng, X. Likun, S. Chengjin, and L. Xiangbo, Electrochemical Characterization for Corrosion Resistance of Plasma-Sprayed Cr2O3 Coating with Sealing, J. Chin. Soc. Corros. Prot., 2012, 32(6), p 473–477
Inspection and Quarantine of PRC. General administration of quality supervision, Standardization administration of the People’s Republic of China, “Method for Testing Antifouling Panels in Shallow Submergence,” GB/T 5370-2007, Standardization administration of the People’s Republic of China (2007)
Inspection and Quarantine of PRC. General administration of quality supervision, Standardization administration of the People’s Republic of China, “Determination for release rate of cupper ions for antifouling paints on ship bottoms,” GB/T 6824-2008, Standardization administration of the People’s Republic of China (2008)
A. Lotz, Marine Coatings: Making Sense of US, State, and Local Mandates of Copper-Based Antifouling Regulations, JCT CoatingsTech, 2016, 13(9), p 50–54
M. Tribou and G. Swain, The Effects of Grooming on a Copper Ablative Coating: A Six Year Study, Biofouling, 2017, 33(6), p 494–504
A. Moridi, S. Hassani-Gangaraj, M. Guagliano, and M. Dao, Cold Spray Coating: Review of Material Systems and Future Perspectives, Surf. Eng., 2014, 30(6), p 369–395
G. Burstein, H. Bi, and G. Kawaley, The Persistence of Inhibition of Copper Corrosion in Tap Water, Electrochim. Acta, 2016, 191, p 247–255
R.J. Ferguson, Modeling Lead and Copper Corrosion and Solubility in Municipal Water Distribution Systems, CORROSION 2017, 2017, NACE International
J. Wu, L. Wang, P. Liu, Y. Liu, B. Chen, Z. Tao, W. He, Electrochemical investigation of thiourea as corrosion inhibitor for copper in acidic solution, in AIP Conference Proceedings (AIP Publishing, 2017), p 020047
D. Chandran, H.K. Ng, H.L.N. Lau, S. Gan, and Y.M. Choo, Investigation of the Effects of Palm Biodiesel Dissolved Oxygen and Conductivity on Metal Corrosion and Elastomer Degradation Under Novel Immersion Method, Appl. Therm. Eng., 2016, 104, p 294–308
C. Demuth, J. Varonier, V. Jossen, R. Eibl, and D. Eibl, Novel Probes for pH and Dissolved Oxygen Measurements in Cultivations from Millilitre to Benchtop Scale, Appl. Microbiol. Biotechnol., 2016, 100(9), p 3853–3863
H. Bi, G. Burstein, B. Rodriguez, and G. Kawaley, Some Aspects of the Role of Inhibitors in the Corrosion of Copper in Tap Water as Observed by Cyclic Voltammetry, Corros. Sci., 2016, 102, p 510–516
P. Broadbridge, B. Bradshaw-Hajek, D. Triadis, Exact non-classical symmetry solutions of Arrhenius reaction–diffusion, in Proceedings of the Royal Society A (The Royal Society, 2015), p. 20150580
G. Hultquist, M. Graham, O. Kodra, S. Moisa, R. Liu, U. Bexell, J. Smialek, Corrosion of copper in distilled water without molecular oxygen and the detection of produced hydrogen. Strålsäkerhetsmyndigheten (2013)
G. Hultquist, M. Graham, J. Smialek, O. Kodra et al., Response to Comment by A. Hedin et al. on “Corrosion of Copper in Distilled Water Without Oxygen and the Detection of Produced Hydrogen, Corros. Sci., 2016, 106, p 306–307
J.H. Michel, I. Richardson, C. Powell, B. Phull, Development of Copper Alloys for Seawater Service from Traditional Application to State-of-the Art Engineering, CORROSION 2017 (NACE International, 2017)
L. Wu, S. Hahn, C. Yan, Modeling of Evolution Process of Edge Over Erosion in Copper CMP Using Frequency Components Algorithm, in Semiconductor Technology International Conference (CSTIC), 2016 China (IEEE, 2016), pp. 1–3
C.H. Hsu and F. Mansfeld, Technical Note: Concerning the Conversion of the Constant Phase Element Parameter Y0 into a Capacitance, Corrosion, 2012, 57(9), p 747–748
S.S. Vaghani, M.M. Patel, and C.S. Satish, Synthesis and Characterization of pH-Sensitive Hydrogel Composed of Carboxymethyl Chitosan for Colon Targeted Delivery of Ornidazole, Carbohyd. Res., 2012, 347(1), p 76
Acknowledgments
The project was achieved with the China Postdoctoral Science Fund, Grand No. 2018M632726, Qingdao Independent Innovation Special Project, Grant No. 16-7-1-2-ZDZXX, National Science Fund for Distinguished Young Scholars, Grand No. 51525903, AoShan talents cultivation program supported by Qingdao National Laboratory for Marine Science and Technology, Grant No. 2017ASTCP-OS09.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, R., Li, X., Wang, J. et al. Antifouling Properties and Release of Dissolved Copper of Cold Spray Cu/Cu2O Coatings for Ships and Steel Structures in Marine Environment. J. of Materi Eng and Perform 27, 5947–5963 (2018). https://doi.org/10.1007/s11665-018-3580-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-018-3580-7