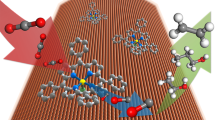
Abstract
The development of an efficient catalytic process that mimics the enzymatic function of alcohol dehydrogenase is critical for using biomass alcohols for both the production of H2 as a chemical energy carrier and fine chemicals under waste-free conditions. Dehydrogenation of alcohol–water mixtures into their corresponding acids with molecular hydrogen as the sole by-product from the reaction can be catalysed by a ruthenium complex with a chelating bis(olefin) diazadiene ligand. This complex, [K(dme)2][Ru(H)(trop2dad)], stores up to two equivalents of hydrogen intramolecularly, and catalyses the production of H2 from alcohols in the presence of water and a base under homogeneous conditions. The conversion of a MeOH–H2O mixture proceeds selectively to CO2/H2 gas formation under neutral conditions, thereby allowing the use of the entire hydrogen content (12% by weight). Isolation and characterization of the ruthenium complexes from these reactions suggested a mechanistic scenario in which the trop2dad ligand behaves as a chemically ‘non-innocent’ co-operative ligand.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Navarro, R. M., Pena, M. A. & Fierro, J. L. G. Hydrogen production reactions from carbon feedstocks: fossil fuels and biomass. Chem. Rev. 107, 3952–3991 (2007).
Gärtner, F. et al. Hydrogen evolution from water/alcohol mixtures: effective in situ generation of an active Au/TiO2 catalyst. ChemSusChem. 5, 530–533 (2012).
Okamoto, Y., Ida, S., Hyodo, J., Hagiwara, H. & Ishihara T. Synthesis and photocatalytic activity of rhodium-doped calcium niobate nanosheets for hydrogen production from a water/methanol system without cocatalyst loading. J. Am. Chem. Soc. 133, 18034–18037 (2011).
Morton, D. & Cole-Hamilton, D. J. Rapid thermal hydrogen production from alcohols catalysed by [Rh(2,2′-bipyridyl)2]CI. Chem. Commun. 248–249 (1987).
Shinoda, S., Itagaki, H. & Saito, Y. Dehydrogenation of methanol in the liquid phase with a homogeneous ruthenium complex catalyst. Chem. Commun. 860–861 (1985).
Smith, T. A., Aplin, R. P. & Maitlis, P. M. The ruthenium-catalysed conversion of methanol into methyl formate. J. Organomet. Chem. 291, C13–C14 (1985).
Fujii, T. & Saito, Y. Catalytic dehydrogenation of methanol with ruthenium complexes. J. Mol. Catal. 67, 185–190 (1991).
Yamakawa, T., Hiroi, M. & Shinoda, S. Catalytic reaction of methanol with a series of ruthenium(II) complexes and the mechanism of the formation of acetic acid from methanol alone. J. Chem. Soc. Dalton Trans. 2265–2269 (1994).
Makita, K., Nomura, K. & Saito, Y. Photocatalytic dehydrogenation of methanol using [IrH(SnCl3)5]3− complex. J. Mol. Catal. 89, 143–150 (1994).
Johnson, T. C., Morris, D. J. & Wills M. Hydrogen generation from formic acid and alcohols using homogeneous catalysts. Chem. Soc. Rev. 39, 81–88 (2010).
Blum, Y. & Shvo, Y. Catalytically reactive (η4-tetracyclone)(CO)2(H)2Ru and related complexes in dehydrogenation of alcohols to esters. J. Organomet. Chem. 282, C7–C10 (1985).
Zhang, J., Leitus, G., Ben-David, Y. & Milstein, D. Facile conversion of alcohols into esters and dihydrogen catalyzed by new ruthenium complexes. J. Am. Chem. Soc. 127, 10840–10841 (2005).
Gunanathan, C., Ben-David, Y. & Milstein, D. Direct synthesis of amides from alcohols and amines with liberation of H2 . Science 317, 790–792 (2007).
Nielsen, M. et al. Hydrogen production from alcohols under mild reaction conditions. Angew. Chem. Int. Ed. 50, 9593–9597 (2011).
Nielsen, M., Junge, H., Kammer, A. & Beller, M. Towards a green process for bulk-scale synthesis of ethyl acetate: efficient acceptorless dehydrogenation of ethanol. Angew. Chem. Int. Ed. 51, 5711–5713 (2012).
Spasyuk, D. & Gusev, D. G. Acceptorless dehydrogenative coupling of ethanol and hydrogenation of esters and imines. Organometallics 31, 5239–5242 (2012).
Trincado, M., Grützmacher, H., Vizza, F. & Bianchini, C. Domino rhodium/palladium-catalyzed dehydrogenation reactions of alcohols to acids by hydrogen transfer to inactivated alkenes. Chem. Eur. J. 16, 2751–2757 (2010).
Trincado, M., Kühlein, K. & Grützmacher, H. Metal–ligand cooperation in the catalytic dehydrogenative coupling (DHC) of polyalcohols to carboxylic acid derivatives. Chem. Eur. J. 17, 11905–11913 (2011).
Annen S. P. et al. A biologically inspired organometallic fuel cell (OMFC) that converts renewable alcohols into energy and chemicals. Angew. Chem. Int. Ed. 49, 7229–7233 (2010).
Bevilacqua, C. et al. Improvement in the efficiency of an organometallic fuel cell by tuning the molecular architecture of the anode electrocatalyst and the nature of the carbon support. Energy Environ. Sci. 5, 8608–8620 (2012).
Wesselbaum, S., vom Stein, T., Klankermayer, J. & Leitner, W. Hydrogenation of carbon dioxide to methanol by using a homogeneous ruthenium–phosphine catalyst. Angew. Chem. Int. Ed. 51, 7499–7502 (2012).
Olah, G. A., Goeppert, A. & Prakash, G. K. S. Beyond Oil and Gas: The Methanol Economy (Wiley-VCH, 2009).
Grasemann, M. & Laurenczy, G. Formic acid as a hydrogen source—recent developments and future trends. Energy Environ. Sci. 5, 8171–8181 (2012).
Himeda, Y. Highly efficient hydrogen evolution by decomposition of formic acid using an iridium catalyst with 4,4-dihydroxy-2,2-bipyridine. Green Chem. 11, 2018–2022 (2009).
Hull, J. F. et al. Reversible hydrogen storage using CO2 and a proton-switchable iridium catalyst in aqueous media under mild temperatures and pressures. Nature Chem. 4, 383–388 (2012).
Lyaskovsky, V. & de Bruin, B. Redox non-innocent ligands: versatile new tools to control catalytic reactions. ACS Catal. 2, 270–279 (2012).
Caulton, K. G. Systematics and future projections concerning redox-noninnocent amide/imine ligands. Eur. J. Inorg. Chem. 435–443 (2012).
Chirik, P. J. & Wieghardt, K. Radical ligands confer nobility on base-metal catalysts. Science 327, 794–795 (2012).
Kaim, W. et al. The 1,4-diazabutadiene/1,2-enediamido non-innocent ligand system in the formation of iridaheteroaromatic compounds: spectroelectrochemistry and electronic structure. J. Organomet. Chem. 695, 1052–1058 (2010).
Puschmann, F. F. et al. Electromeric rhodium radical complexes. Angew. Chem. Int. Ed. 49, 385–389 (2010).
Bally, T. Isomerism: the same but different. Nature Chem. 2, 165–166 (2010).
Knijnenburg, Q., Gambarottab, S. & Budzelaar, P. H. M. Ligand-centered reactivity in diiminepyridine complexes. Dalton Trans. 5442–5448 (2006).
Keene, F. R. Metal-ion promotion of the oxidative dehydrogenation of coordinated amines and alcohols. Coord. Chem. Rev. 187, 121–149 (1999).
Greulich, S., Klein, A., Knoedler, A. & Kaim, W. Qualitatively different reactivities of hydride reagents toward [(α-diimine)(η5-C5Me5)ClIr]+ cations: substitution, electron transfer (reduction), or stepwise hydrogenation. Organometallics 21, 765–769 (2002).
Mikhailine, A. A., Maishan, M. I., Lough, A. J. & Morris, R. H. The mechanism of efficient asymmetric transfer hydrogenation of acetophenone using an iron(II) complex containing an (S,S)-Ph2PCH2CH=NCHPhCHPhN=CHCH2PPh2 ligand: partial ligand reduction is the key. J. Am. Chem. Soc. 134, 12266–12280 (2012).
De Bruin, B. & Hetterscheid, D. G. H. Paramagnetic (alkene)Rh and (alkene)Ir complexes: metal or ligand radicals? Eur. J. Inorg. Chem. 211–230 (2007).
Defieber, C., Grützmacher, H. & Carreira, E. Chiral olefins as steering ligands in asymmetric catalysis. Angew. Chem. Int. Ed. 47, 4482–4502 (2008).
Breher, F. et al. TROPDAD: a new ligand for the synthesis of water-stable paramagnetic [16 + 1]-electron rhodium and iridium complexes. Chem. Eur. J. 9, 3859–3866 (2003).
Maire, P., Breher, F. & Grützmacher, H. Diamido rhodates(1−). Angew. Chem. Int. Ed. 44, 6325–6329 (2005).
Askevold, B., Khusniyarov, M., Herdtweck, E., Meyer, K. & Schneider, S. A square-planar ruthenium(II) complex with a low-spin configuration. Angew. Chem. Int. Ed. 49, 7566–7569 (2010).
Watson, L. A., Ozerov, O. V., Pink, M. & Caulton, K. G. A triplet state as a response to a 14-valence electron configuration. J. Am. Chem. Soc. 125, 8426–8427 (2003).
Hiraki, K., Nanoka, A., Matsunga, T. & Kawano, H. Reactions of [RuClH(CO)(PPh3)3] with 2-methyl-2-propen-1-ol. Reversible insertion: β-elimination, and reductive elimination on a 3-hydroxy-2-methylpropyl-C1,O-ruthenium(II) complex. J. Organomet. Chem. 574, 121–132 (1999).
Gottschalk-Gaudig, T., Huffman, J. C., Gerard, H. G., Eisenstein, O. & Caulton, K. G. Unsaturated Ru(0) species with a constrained bis-phosphine ligand: [Ru(CO)2(tBu2PCH2CH2PtBu2)]2. Comparison to [Ru(CO)2(PtBu2Me)2]. Inorg. Chem. 39, 3957–3962 (2000).
Acknowledgements
This work was supported by the Schweizer Nationalfonds (SNF), Eidgenössische Hochschule Zürich and the joint SNF/Deutsche Forschungsgemeinschaft research project ‘Unconventional Approaches to the Activation of Dihydrogen’ (FOR1175). H.G. thanks P. Edwards (University of Oxford) and Lotus Cars, in particular, for inspiration.
Author information
Authors and Affiliations
Contributions
G.S-Q. performed the X-ray diffraction measurements with single crystals. All other authors planned and performed the experiments. R.E.R-L., M.T. and H.G. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1450 kb)
Supplementary information
Crystallographic data for compound 2 (CIF 28 kb)
Supplementary information
Crystallographic data for compound 3 (CIF 26 kb)
Supplementary information
Crystallographic data for compound 5 (CIF 15 kb)
Supplementary information
Crystallographic data for compound 6 (CIF 28 kb)
Supplementary information
Crystallographic data for compound 7 (CIF 28 kb)
Rights and permissions
About this article
Cite this article
Rodríguez-Lugo, R., Trincado, M., Vogt, M. et al. A homogeneous transition metal complex for clean hydrogen production from methanol–water mixtures. Nature Chem 5, 342–347 (2013). https://doi.org/10.1038/nchem.1595
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1595
This article is cited by
-
Sustainable production of hydrogen with high purity from methanol and water at low temperatures
Nature Communications (2022)
-
General heterostructure strategy of photothermal materials for scalable solar-heating hydrogen production without the consumption of artificial energy
Nature Communications (2022)
-
Reversible interconversion between methanol-diamine and diamide for hydrogen storage based on manganese catalyzed (de)hydrogenation
Nature Communications (2020)
-
Ethylene glycol as an efficient and reversible liquid-organic hydrogen carrier
Nature Catalysis (2019)
-
N-formylation of amines using methanol as a potential formyl carrier by a reusable chromium catalyst
Communications Chemistry (2019)