Abstract
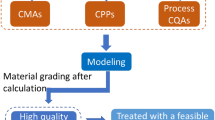
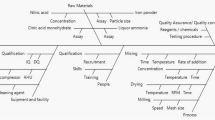
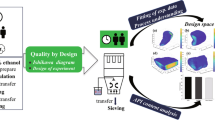
This paper was designed to assess the value of quality by design (QbD) to improve the manufacturing process understanding of botanical drug products. Ethanol precipitation, a widely used unit operation in the manufacture of botanical drug products was employed to illustrate the use of QbD, taking the process of danshen (the dry root of Salvia miltiorrhiza Bunge) as an example. The recovery of four active pharmaceutical ingredients (APIs) and the removal of saccharides were used to represent the performance of ethanol precipitation. Potentially critical variables, including density of concentrate, ethanol consumption, and settling temperature were identified through risk assessment methods. Design of experiments (DOE) was used to evaluate the effects of the potentially critical factors on the performance of ethanol precipitation. It was observed that higher density of concentrate leads to higher removal of saccharides, but results in lower recovery of APIs. With the rise of ethanol consumption, the recovery of different APIs behaves in different ways. A potential design space of ethanol precipitation operation was established through DOE studies. The results in this work facilitate the enhanced understanding of the relationships between multiple factors (material attributes and process parameters) and the performance of ethanol precipitation. This case study demonstrated that QbD is a powerful tool to develop manufacturing process of botanical drug products.







Similar content being viewed by others
References
Yang L, Wang Y, Wang L, Xiao H, Wang Z, Hu Z. Rapid quantification of iridoid glycosides analogues in the formulated Chinese medicine Longdan Xiegan Decoction using high-performance liquid chromatograph coupled with mass spectromentry. J Chromatogr A. 2009;1216:2098–103.
ICH Q8 (R2). Pharmaceutical development. 2009. http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html. Accessed 18 May 2012.
Basalious EB, El-Sebaie W, El-Gazayerly O. Application of pharmaceutical QbD for enhancement of the solubility and dissolution of a class II BCS drug using polymeric surfactants and crystallization inhibitors: development of controlled-release tablets. AAPS Pharm Sci Tech. 2011;12(3):799–810.
Yu LX. Pharmaceutical quality by design: product and process development, understanding, and control. Pharm Res. 2008;25(4):781–91.
Zhang X, Lionberger RA, Davit BM, Yu LX. Utility of physiological based absorption modeling in implementing quality by design in drug development. AAPS J. 2011;13(1):59–71.
Al-Hallak MHDK, Azarmi S, Xu Z, Maham Y, Löbenberg R. Isothermal microcalorimetry as a quality by design tool to determine optimal blending sequences. AAPS J. 2010;12(3):417–23.
Chen Y, Jin Y, Cai S, Cheng Y, Qu H. Study Progresses: TCM ethanol precipitation techniques and associated equipment. World Sci Technol. 2007;9(5):16–9.
Gong X, Wang S, Qu H. Comparison of two separation technologies applied in the manufacture of botanical injections: second ethanol precipitation and solvent extraction. Ind Eng Chem Res. 2011;50:7542–8.
Koh GY, Chou G, Liu Z. Purification of a water extract of Chinese sweet tea plant (Rubus suavissimus S. Lee) by alcohol precipitation. J Agric Food Chem. 2009;57:5000–6.
Schmourlo G, Mendonça-Filho RR, Alviano CS, Costa SS. Screening of antifungal agents using ethanol precipitation and bioautography of medicinal and food plants. J Ethnopharmacol. 2005;96:563–8.
Wang X, Morris-Natscbke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev. 2007;27(1):133–48.
Li YG, Song L, Liu M, Hu ZB, Wang ZT. Advancement in analysis of Salviae miltiorrhizae Radix et Rhizoma (Danshen). J Chromatogr A. 2009;1216:1941–53.
Zhou L, Zuo Z, Chow MSS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–59.
Lau KM, Lai KK, Liu CL, Tam JCW, To MH, Kwok HF, et al. Synergistic interaction between Astragali Radix and Rehmanniae Radix in a Chinese herbal formula to promote diabetic wound healing. J Ethnopharmacol. 2012;141:250–6.
Izumi E, Ueda-Nakamura T, Veiga VF, Pinto AC, Nakamura CV. Terpenes from Copaifera demonstrated in vitro antiparasitic and synergic activity. J Med Chem. 2012;55:2994–3001.
Herranz-Lopez M, Fernandez-Arroyo S, Perez-Sanchez A, Barrajon-Catalan E, Beltran-Debon R, Menedez JA, et al. Synergism of plant-derived polyphenols in adipogenesis: perspectives and implications. Phytomedicine. 2012;19:253–61.
Li H, Song F, Zheng Z, Liu Z, Liu S. Characterization of saccharides and phenolic acids in the Chinese herb Tanshen by ESI-FT-ICR-MS and HPLC. J Mass Spectrom. 2008;43:1545–52.
Falcone PM, Tagliazucchi D, Verzelloni E, Giudici P. Sugar conversion induced by the application of heat to grape must. J Agric Food Chem. 2010;58:8680–91.
Severin I, Dumont C, Jondeau-Cabaton A, Graillot V, Chagnon MC. Genotoxic activities of the food contaminant 5-hydroxymethylfurfural using different in vitro bioassays. Toxicol Lett. 2010;192:189–94.
Adam S, Suzzi D, Radeke C, Khinast JG. An integrated quality by design (QbD) approach towards design space definition of a blending unit operation by discrete element method (DEM) simulation. Eur J Pharm. 2011;42:106–15.
ICH Q9. Quality risk management. 2005. http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html. Accessed 18 May 2012.
Commission CP. Chinese Pharmacopoeia. Beijing: China Medical Science Press; 2010. p. 907.
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–6.
Lepore J, Spavins J. PQLI design space. J Pharm Innov. 2008;3:79–87.
Boukouvala F, Muzzio FJ, Ierapetritou MG. Design space of pharmaceutical processes using data-driven-based methods. J Pharm Innov. 2010;5:119–37.
Huang J, Kaul G, Cai C, Chatlapalli R, Hernandez-Abad P, Ghosh K, Nagi A. Quality by design case study: an integrated multivariate approach to drug product and process development. Int J Pharm. 2009;382:23–32.
Zhang L, Gong X, Wang Y, Qu H. Solubilities of protocatechuic aldehyde, caffeic acid, d-galactose, and d-raffinose pentahydrate in ethanol-water solutions. J Chem Eng Data. 2012;57:2018–22.
Alves LA, Silva JBA, Giulietti M. Solubility of d-glucose in water and ethanol/water mixtures. J Chem Eng Data. 2007;52(6):2166–70.
Bouchard A, Hofland GW, Witkamp GJ. Properties of sugar, polyol, and polysaccharide water-ethanol solutions. J Chem Eng Data. 2007;52(5):1838–42.
Gong X, Wang S, Qu H. Solid–liquid equilibria of d-glucose, d-fructose and sucrose in the mixture of ethanol water from 273.2 K to 293.2 K. Chin J Chem Eng. 2011;19(2):1–6.
Macedo EA, Peres AM. Thermodynamics of ternary mixtures containing sugars. SLE of d-fructose in pure and mixed solvents. Comparison between modified UNIQUAC and modified UNIFAC. Ind Eng Chem Res. 2001;40(21):4633–40.
Kapsi SG, Castro LD, Muller FX, Wrzosek TJ. Development of a design space for a unit operation: illustration using compression-mix blending process for the manufacture of a tablet dosage form. J Pharm Innov. 2012;7:19–29.
ICH Q10. Pharmaceutical quality system. 2008. http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html. Accessed 18 May 2012.
Huang H, Qu H. In-line monitoring of alcohol precipitation by near-infrared spectroscopy in conjunction with multivariate batch modeling. Anal Chem Acta. 2011;707:47–56.
Acknowledgments
This work was financially supported by the China International Science and Technology Cooperation Project (2010DFB33630) and Zhejiang Provincial Natural Science Foundation of China (LQ12H29004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Yan, B., Gong, X. et al. Application of Quality by Design to the Process Development of Botanical Drug Products: A Case Study. AAPS PharmSciTech 14, 277–286 (2013). https://doi.org/10.1208/s12249-012-9919-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-012-9919-8