Abstract
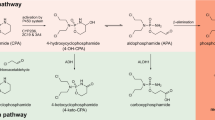
The antitumor activity of shikonin derivatives is largely dependent on the generation of superoxide radicals and the alkylation activity of their naphthoquinone moiety. However, our recent study showed that 1,4-dioxime-5,8-dimethoxynaphthalene (DMAKO-05), a novel shikonin derivative, displayed more potential antitumor activity and less toxicity compared to fluorouracil (5-FU) both in vitro and in vivo, even though the hydroxyl and carbonyl groups of its naphthoquinone structure were shielded. To understand the underlying mechanisms, we investigated the metabolism of DMAKO-05 in rat liver microsomes. The kinetic analysis indicated that DMAKO-05 underwent a biphasic metabolism in rat liver microsomes. The inhibition experiments showed that CYP1A and CYP3A were the major enzymes in the metabolism of DMAKO-05, along with partial contribution from CYP2A. In addition, the structures of eight DMAKO-05 metabolites, which were characterized by accurate mass and MS/MS fragmentograms, implied that DMAKO-05 was mainly metabolized through the oxygenation of its naphthoquinone nucleus and the hydrolysis of its side chain, instead of the oxidation of hydroxyimine to ketone. Therefore, DMAKO-05 should not be considered as a traditional naphthoquinone prodrug.






Similar content being viewed by others
REFERENCES
Hübotter F. Beiträge zur Kenntnis der chinesischen sowie der tibetisch-mongolischen Pharmakologie. Urban & Schwarzenberg; 1913.
Kim H, Ahn BZ. Antitumor effects of acetylshikonine and some synthesized naphthazarins on L1210 and S-180 systems. Yakhak Hoeji. 1990;34(4):262–6.
Miller MG, Rodgers A, Cohen GM. Mechanisms of toxicity of naphthoquinones to isolated hepatocytes. Biochem Pharmacol. 1986;35(7):1177–84. doi:10.1016/0006-2952(86)90157-7.
Moore HW. Bioactivation as a model for drug design bioreductive alkylation. Science. 1977;197(4303):527–32. doi:10.1126/science.877572.
Zhou W, Zhang X, Xiao L, Ding J, Liu QH, Li SS. Semi-synthesis and antitumor activity of 6-isomers of 7, 8-O-dimethyl acylshikonin derivatives. Eur J Med Chem. 2011;46(8):3420–7. doi:10.1016/j.ejmech.2011.05.006.
Papageorgiou VP, Assimopoulou AN, Couladouros EA, Hepworth D, Nicolaou KC. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew Chem Int Ed. 1999;38(3):270–301. doi:10.1002/(SICI)1521-3773(19990201)38:3<270::AID-ANIE270>3.0.CO;2-0.
Wang RB, Zhou SS, Jiang HDGL, Zheng XG, Zhou W, Li SS. An efficient multigram synthesis of alkannin and shikonin. Eur J Org Chem. 2012;2012(7):1373–9. doi:10.1002/ejoc.201101505.
Feng LS, Lv K, Liu ML, Wang S, Zhao J, You XF, et al. Synthesis and in vitro antibacterial activity of gemifloxacin derivatives containing a substituted benzyloxime moiety. Eur J Med Chem. 2012;55:125–36. doi:10.1016/j.ejmech.2012.07.010.
Zhou W, Peng Y, Li SS. Semi-synthesis and anti-tumor activity of 5,8-O-dimethyl acylshikonin derivatives. Eur J Med Chem. 2010;45(12):6005–11. doi:10.1016/j.ejmech.2010.09.068.
Sistla R, Tata VSSK, Kashyap YV, Chandrasekar D, Diwan PV. Development and validation of a reversed-phase HPLC method for the determination of ezetimibe in pharmaceutical dosage forms. J Pharm Biomed Anal. 2005;15(3–4):517–22. doi:10.1016/j.jpba.2005.04.026.
Messiano GB, Santos RAS, Ferreira LDS, Simões RA, Jabor VAP, Kato MJ, et al. In vitro metabolism study of the promising anticancer agent the lignan (−)-grandisin. J Pharm Biomed Anal. 2013;72:240–4. doi:10.1016/j.jpba.2012.08.028.
Newton DJ, Wang RW, Lu AYH. Cytochrome P450 inhibitors. Evaluation of specificities in the in vitro metabolism of therapeutic agents by human liver microsomes. Drug Metab Dispos. 1994;23(1):154–8.
Guidance for Industry: bioanalytical method validation. Center for Drug Evaluation and Research. United States Food and Drug Administration; 2001.
Causon R. Validation of chromatographic methods in biomedical analysis viewpoint and discussion. J Chromatogr B Biomed Appl. 1997;689(1):175–80.
Ekins S, Ring BJ, Grace J, McRobie-Belle DJ, Wrighton SA. Present and future in vitro approaches for drug metabolism. J Pharmacol Toxicol Methods. 2000;44(1):313–24. doi:10.1016/S1056-8719(00)00110-6.
Fasinu P, Bouic PJ, Rosenkranz B. Liver-based in vitro technologies for drug biotransformation studies–a review. Curr Drug Metab. 2012;13(2):215–24. doi:10.2174/138920012798918426.
Zhang DL, Zhu MS, Humphreys WG. Drug metabolism in drug design and development: basic concepts and practice. 2007;426. doi: 10.1002/9780470191699.
Kharasch ED, Labroo R. Metabolism of ketamine stereoisomers by human liver microsomes. Anesthesiology. 1992;77(6):1201–7. doi:10.1097/00000542-199212000-00022.
Shi Q, Greenhaw J, Salminen WF. Inhibition of cytochrome P450s enhances (+)-usnic acid cytotoxicity in primary cultured rat hepatocytes. J Appl Toxicol. 2013. doi:10.1002/jat.2892.
Chiba M, Hensleigh M, Lin JH. Liver and intestinal metabolism of indinavir, an HIV protease inhibitor, in rat and human microsomes. Major role of CYP3A. Biochem Pharmacol. 1997;53(8):1187–95. doi:10.1016/S0006-2952(97)00100-7.
Li H, Luo S, Zhou T. Studies on in vitro metabolism of shikonin. Phytother Res. 1999;13(3):236–8.
Kumpulainen H, Mähönen N, Laitinen ML, Jaurakkajärvi M, Raunio H, Juvonen RO, et al. Evaluation of hydroxyimine as cytochrome P450-selective prodrug structure. J Med Chem. 2006;49(3):1207–11. doi:10.1021/jm0510124.
Roberts AB, Lamb LC, Sporn MB. Metabolism of all-trans-retinoic acid in hamster liver microsomes: oxidation of 4-hydroxy- to 4-keto-retinoic acid. Arch Biochem Biophys. 1980;199(2):374–83. doi:10.1016/0003-9861(80)90293-3.
ACKNOWLEDGMENTS
This work was supported by the Shanghai Committee of Science and Technology, China (Grant No. 12431900602), and the State Key Laboratory Cultivation Base for the Chemistry and Molecular Engineering of Medicinal Resources, Ministry of Science and Technology of China (CHEMR2012-B08). We also thank Vijaykanth Pagadala and Tim O’Leary for their critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xu Zhang and Ru-Bing Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, X., Wang, RB., Zhou, W. et al. Antitumor Activity of DMAKO-05, a Novel Shikonin Derivative, and Its Metabolism in Rat Liver Microsome. AAPS PharmSciTech 16, 259–266 (2015). https://doi.org/10.1208/s12249-014-0217-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0217-5