Abstract
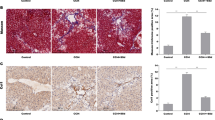
A mygdalin is one of the nitrilosides that was widely used to treat cancer, inhibit liver fibrosis. In the present study, the aim was to determine the antifibrotic potential of amygdalin and examine its mechanisms of action in vitro. Firstly, we found amygdalin significantly inhibited HSC-T6 cells proliferation. Both mRNA and protein of transforming growth factor-β (TGF-β) were decreased in HSC-T6 cells during amygdalin treatment. Secondly, to investigate functional role of TGF-β, both TGF-β over-expression vector and siRNA against TGF-β were transfected into HSC-T6 cells respectively. The results showed that over-expression of TGF-β promoted proliferation of HSC-T6 cells, whereas TGF-β knockdown inhibited cell viability. Moreover, our data even indicated that TGF-β could promote cell proliferation independent of amygdalin treatment. Finally, we found amygdalin could inhibit expression of the classical fibrotic factor αSMA, which suggested an antifibrotic effect of amygdalin. While the TGF-β antagonized anti-fibrotic effect of amygdalin. To assess the mechanisms, we examined expression of CTGF in cultured HSC-T6 cells. Our results showed that CTGF was down-regulated in HSCT6 cell treated by amygdalin, but was up-regulated when exogenous TGF-β introduced. As CTGF was one of the downstream factors in the TGF-β pathway. These might suggest that amygdalin inhibited HSC-T6 cells proliferation and fibrosis via TGF-β/CTGF pathway.
Similar content being viewed by others
References
Cho, A. et al. Detection of abnormally high amygdalin content in food by an enzyme immunoassay. Mol Cells 21:308–313 (2006).
Hwang, H. J. et al. Antinociceptive effect of amygdalin isolated from Prunus armeniaca on formalin-induced pain in rats. Biol Pharm Bull 31:1559–1564 (2008).
Chang, H. K. et al. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol Pharm Bull 29:1597–1602 (2006).
Fukuda, T. et al. Anti-tumor promoting effect of glycosides from Prunus persica seeds. Biol Pharm Bull 26:271–300 (2003).
Mirmiranpour, H. et al. Amygdalin inhibits angiogenesis in the cultured endothelial cells of diabetic rats. Indian J Pathol Microbiol 55:211–214 (2012).
Lee, U. E. & Friedman, S. L. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol 25:195–206 (2011).
Inagaki, Y. & Okazaki, I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut 56:284–292 (2007).
Limaye, P. B. et al. Mechanisms of hepatocyte growth factor-mediated and epidermal growth factor-mediated signaling in transdifferentiation of rat hepatocytes to biliary epithelium. Hepatology 47:1702–1713 (2008).
Date, M. et al. Modulation of transforming growth factor beta function in hepatocytes and hepatic stellate cells in rat liver injury. Gut 46:719–724 (2000).
Yoshida, K. & Matsuzaki, K. Differential Regulation of TGF-beta/Smad Signaling in Hepatic Stellate Cells between Acute and Chronic Liver Injuries. Front Physiol 3:53 (2012).
Yoshida, K. et al. Transforming growth factor-beta and platelet-derived growth factor signal via c-Jun N-terminal kinase-dependent Smad2/3 phosphorylation in rat hepatic stellate cells after acute liver injury. Am J Pathol 166:1029–1039 (2005).
Williams, E. J., Gaca, M. D., Brigstock, D. R., Arthur, M. J. & Benyon, R. C. Increased expression of connective tissue growth factor in fibrotic human liver and in activated hepatic stellate cells. J Hepatol 32:754–761 (2000).
Huang, G. & Brigstock, D. R. Regulation of hepatic stellate cells by connective tissue growth factor. Front Biosci (Landmark Ed) 17:2495–2507 (2012).
Abou-Shady, M. et al. Connective tissue growth factor in human liver cirrhosis. Liver 20:296–304 (2000).
Torok, N. J. Recent advances in the pathogenesis and diagnosis of liver fibrosis. J Gastroenterol 43:315–321 (2008).
Puche, J. E., Saiman, Y. & Friedman, S. L. Hepatic stellate cells and liver fibrosis. Compr Physiol 3:1473–1492 (2013).
Zhang, L. et al. Danshensu inhibits acetaldehyde-induced proliferation and activation of hepatic stellate cell-T6. Zhong Xi Yi Jie He Xue Ba 10:1155–1161 (2012).
Guo, J., Wu, W., Sheng, M., Yang, S. & Tan, J. Amygdalin inhibits renal fibrosis in chronic kidney disease. Mol Med Rep 7:1453–1457 (2013).
Xu, H. G. et al. Expression of ectonucleotide pyrophosphatase-1 in end-plate chondrocytes with transforming growth factor beta 1 siRNA interference by cyclic mechanical tension. Chin Med J 20:3886–3890 (2013).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408 (2001).
Wang, W. et al. Signaling mechanism of TGF-beta1 in prevention of renal inflammation:role of Smad7. J Am Soc Nephrol 16:1371–1383 (2005).
Ha, M. H. et al. Effect of interferon-gamma on hepatic stellate cells stimulated by acetaldehyde. Hepatogastroenterology 84:1059–65 (2008).
Hernandez-Gea, V. & Friedman, S. L. Pathogenesis of liver fibrosis. Annu Rev Pathol 6:425–456 (2011).
Wells, R. G. The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J Clin Gastroenterol 39:S158–161 (2005).
Zardi, E. M. et al. New therapeutic approaches to liver fibrosis:a practicable route? Curr Med Chem 15:1628–1644 (2008).
Gressner, A. M. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J Hepatol 22:28–36 (1995).
Lipson, K. E., Wong, C., Teng, Y. & Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 5:S24 (2012).
Gressner, O. A. & Gressner, A. M. Connective tissue growth factor:a fibrogenic master switch in fibrotic liver diseases. Liver Int 28:1065–1079 (2008).
Friedman, S. L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 275:2247–2250 (2000).
Potter, J. J. & Mezey, E. Acetaldehyde increases endogenous adiponectin and fibrogenesis in hepatic stellate cells but exogenous adiponectin inhibits fibrogenesis. Alcohol Clin Exp Res 31:2092–2100 (2007).
Liu, Y. et al. Transforming growth factor-beta (TGFbeta)-mediated connective tissue growth factor (CTGF) expression in hepatic stellate cells requires Stat3 signaling activation. J Biol Chem 288:30708–30719 (2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, H., Li, L., Tang, J. et al. Amygdalin inhibits HSC-T6 cell proliferation and fibrosis through the regulation of TGF-β/CTGF. Mol. Cell. Toxicol. 12, 265–271 (2016). https://doi.org/10.1007/s13273-016-0031-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-016-0031-0